 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route
-
-
31844-92-5
2-chloro-N-phenyl-3-oxobutanamide

-
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With potassium carbonate; para-thiocresol In acetonitrile at 20℃; for 1h; Reagent/catalyst; | 100% |
| Multi-step reaction with 2 steps 1: potassium carbonate / acetonitrile / 6 h / 20 °C 2: para-thiocresol; potassium carbonate / acetonitrile / 0.33 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| In propan-1-ol at 30 - 50℃; for 5h; Solvent; Temperature; Autoclave; Large scale; | 98% |
| In cyclohexane at 25℃; Equilibrium constant; Rate constant; | |
| With benzene |

| Conditions | Yield |
|---|---|
| With sodium acetate In tetrahydrofuran for 24h; Reflux; | 98% |
| In water for 2.5h; Reflux; Green chemistry; | 94% |
| In 5,5-dimethyl-1,3-cyclohexadiene Reflux; | 82% |

| Conditions | Yield |
|---|---|
| for 0.0833333h; Acylation; microwave irradiation; | 97% |
| for 0.1h; Microwave irradiation; | 94% |
| With silver trifluoromethanesulfonate In nitromethane at 80℃; for 8h; | 90% |
-
-
59846-49-0
3-(methylamino)-N-phenylbut-2-enamide

-
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-(methylamino)-N-phenylbut-2-enamide With trifluoroacetic acid In dichloromethane at 20℃; for 0.5h; Stage #2: With sodium hydrogencarbonate In dichloromethane Further stages.; | 93% |
-
-
154867-20-6
3-phenylamino-2-[(phenylimino)methyl]acrylonitrile

-
-
5394-63-8
2,2,6-trimethyl-4H-1,3-dioxin-4-one

-
A
-
1352630-34-2
5-acetyl-6-oxo-1-phenyl-1,6-dihydropyridine-3-carbonitrile

-
B
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| In toluene at 110℃; for 0.166667h; Inert atmosphere; | A 91% B n/a |


-
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With chloro[1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene]copper(I); oxygen In tetrahydrofuran at 25℃; for 16h; Reagent/catalyst; Sealed tube; Inert atmosphere; | 91% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen In toluene at 25℃; for 48h; | 30% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol In acetonitrile at 80℃; for 12h; Sealed tube; Green chemistry; | 88% |
| With natural kaolinitic clay In toluene Heating; | 60% |
| yttria-zirconia based Lewis acid catalyst In toluene for 3h; Acylation; Heating; | 59% |
| In toluene for 24h; Heating / reflux; | 50.3% |
| Heating; |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol In acetonitrile at 80℃; for 12h; Sealed tube; Green chemistry; | 87% |

| Conditions | Yield |
|---|---|
| In xylene Heating; | 83% |
| In toluene at 150℃; for 14h; | 56% |
| In toluene Reflux; |

| Conditions | Yield |
|---|---|
| In benzene at 100℃; | 80% |

-
A
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; acetonitrile at 80℃; for 0.166667h; | A 4% B 70% |
| With hydrogenchloride In water; acetonitrile at 80℃; for 0.166667h; Product distribution; Mechanism; other 1,4-oxathiin-3-carboxamides, other reagent: H2SO4, other solvents: benzene, toluene, dioxane, THF, EtOH, various reagent concentration, various reaction temperature and time; | A 4% B 70% |
| With hydrogenchloride In water; acetonitrile at 80℃; for 0.166667h; other reagent: H2SO4; | A 4% B 70% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; N,N,N,N,-tetramethylethylenediamine; potassium carbonate In tetrahydrofuran for 12h; Inert atmosphere; Reflux; | A 70% B 25% |
| With copper(l) iodide; N,N,N,N,-tetramethylethylenediamine; potassium carbonate In 1,4-dioxane for 3h; Inert atmosphere; Reflux; | A 38% B 40% |

| Conditions | Yield |
|---|---|
| With chloro[1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene]copper(I) In toluene at 25℃; for 2h; Inert atmosphere; | A 30% B 70% |

-
-
1058721-69-9
C10H10BrNO

-
A
-
1058721-80-4
C10H9NO

-
B
-
102-01-2
N-phenylacetoacetamide

-
C
-
179242-29-6
Buta-2,3-dien-anilid

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 2-(2-methyl-1-oxopropyl)cyclohexanone; potassium carbonate In tetrahydrofuran for 16h; Inert atmosphere; Reflux; | A 69% B 6% C 9% |
| With copper(l) iodide; potassium carbonate In tetrahydrofuran for 16h; Inert atmosphere; Reflux; | A 17% B 63% C 16% |
| With copper(l) iodide; potassium carbonate; L-proline In tetrahydrofuran for 16h; Inert atmosphere; Reflux; | A 25% B 23% C 30% |


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0) In toluene at 60℃; under 760.051 Torr; for 6h; | 67% |


-
A
-
102-01-2
N-phenylacetoacetamide

-
B
-
20972-43-4, 21650-65-7, 495-48-7
trans-azoxybenzene

| Conditions | Yield |
|---|---|
| With water; copper dichloride In tetrahydrofuran at 30℃; for 48h; Inert atmosphere; | A 18% B 65% |

| Conditions | Yield |
|---|---|
| With chloro[1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene]copper(I) In toluene at 25℃; for 2h; Inert atmosphere; | A 35% B 65% |

-
-
31844-92-5
2-chloro-N-phenyl-3-oxobutanamide

-
-
106-45-6
para-thiocresol

-
B
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 0.666667h; | A 56% B 27% |
| With potassium carbonate In acetonitrile at 20℃; for 6h; | A 40% B 45% |

-
-
201230-82-2
carbon monoxide

-
-
123-54-6
acetylacetone

-
-
622-37-7
Phenyl azide

-
A
-
102-01-2
N-phenylacetoacetamide

-
B
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| With palladium dichloride In N,N-dimethyl acetamide at 70℃; for 12h; Schlenk technique; | A 31% B 53% |

-
-
59887-23-9
(Z)-3-(benzylamino)-N-phenylbut-2-enamide

-
A
-
1002555-74-9
3-oxo-2,5-dimethyl-1-benzyl-N2,N4-diphenyl-2,3-dihydro-1H-pyrrole-2,4-dicarboxylic acid amide

-
B
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid; bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 4.5h; | A 47% B 21% |
-
-
97-94-9
triethyl borane

-
-
103-71-9
phenyl isocyanate

-
-
7087-68-5
N-ethyl-N,N-diisopropylamine

-
C
-
101-99-5
N-carboethoxyaniline

-
D
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With air at 20℃; for 14h; Further byproducts.; | A 4% B 43% C 10% D 23% |

-
-
34132-56-4
3-phenyl-3,4-dihydro-6-methyl-2H-1,3-oxazine-2,4-dione

-
-
141-43-5
ethanolamine

-
A
-
102-01-2
N-phenylacetoacetamide

-
B
-
38756-99-9
1-(2-hydroxyethyl)-6-methyl-3-phenyl-2,4(1H,3H)-pyrimidinedione

| Conditions | Yield |
|---|---|
| With ethanol at 95 - 100℃; for 2h; | A 15% B 35% |


| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; palladium diacetate; acetic acid at 80℃; for 6h; | 34% |
-
-
141-97-9
ethyl acetoacetate

-
-
62-53-3
aniline

-
A
-
102-01-2
N-phenylacetoacetamide

-
B
-
102-07-8
bis(diphenyl)urea

| Conditions | Yield |
|---|---|
| for 0.0833333h; Microwave irradiation; | A 30% B 1.81 g |

-
-
34132-56-4
3-phenyl-3,4-dihydro-6-methyl-2H-1,3-oxazine-2,4-dione

-
A
-
102-01-2
N-phenylacetoacetamide

-
B
-
38756-99-9
1-(2-hydroxyethyl)-6-methyl-3-phenyl-2,4(1H,3H)-pyrimidinedione

| Conditions | Yield |
|---|---|
| With ethanolamine In N,N-dimethyl-formamide at 95 - 100℃; for 2h; | A 28% B 18% |
-
-
64-17-5
ethanol

-
-
49679-88-1
3-methylamino-but-2-enoic acid anilide

-
A
-
102-01-2
N-phenylacetoacetamide

-
B
-
74-89-5
methylamine

-
-
141-97-9
ethyl acetoacetate

-
-
62-53-3
aniline

-
A
-
102-01-2
N-phenylacetoacetamide

-
B
-
6287-35-0
ethyl 3-anilinocrotonate

-
-
480-96-6
benzofurazan oxide

-
-
102-01-2
N-phenylacetoacetamide

-
-
31983-89-8
2-N-Phenylcarbamyl-3-Methylquinoxaline-di-N-Oxide

| Conditions | Yield |
|---|---|
| Stage #1: benzofurazan oxide; N-phenylacetoacetamide In isopropyl alcohol at 60℃; for 0.5h; Stage #2: With calcium hydroxide In isopropyl alcohol at 60℃; | 100% |
| With 3 A molecular sieve In methanol for 24h; Ambient temperature; | 88% |
| In ethanol | 88% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol for 24h; Biginelli pyrimidine synthesis; Reflux; | 100% |
| With cobalt(II) nitrate hexahydrate In ethanol at 20℃; for 0.666667h; | 90% |
| With hydrogenchloride In ethanol for 3h; Heating; | 65% |
| With hydrogenchloride; aluminum (III) chloride In methanol; water Reflux; |

-
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With trimethylamine at 20℃; under 375.03 Torr; for 12h; | 100% |

-
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-(4,6-dimethyl-3-cyano-2-pyridinylthio)benzenediazonium nitrate; N-phenylacetoacetamide for 0.0833333h; grinding; Stage #2: With trimethylamine at 20℃; under 375.038 Torr; for 12h; Further stages.; | 100% |
-
-
75-15-0
carbon disulfide

-
-
102-01-2
N-phenylacetoacetamide

-
-
77-78-1
dimethyl sulfate

-
-
145508-18-5
2-[bis(methylsulfanyl)methylidene]-3-oxo-N-phenylbutanamide

| Conditions | Yield |
|---|---|
| Stage #1: N-phenylacetoacetamide With tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide for 0.5h; Stage #2: carbon disulfide In N,N-dimethyl-formamide at 20℃; for 2h; Stage #3: dimethyl sulfate In N,N-dimethyl-formamide at 20℃; for 4.5h; | 100% |
| Stage #1: N-phenylacetoacetamide With tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: carbon disulfide In N,N-dimethyl-formamide at 20℃; for 2h; Stage #3: dimethyl sulfate In N,N-dimethyl-formamide at 20℃; for 4.5h; | 96% |

-
-
102-01-2
N-phenylacetoacetamide

-
-
28144-70-9
anthranilic acid amide

-
-
81038-81-5
2-methyl-2-(N-phenylcarbamoyl)methyl-1,2,3,4-tetrahydroquinazolin-4-one

| Conditions | Yield |
|---|---|
| With α-chymotrypsin In ethanol at 60℃; for 40h; Enzymatic reaction; | 99% |
| at 100℃; for 4h; under reduced pressure; | 55% |
-
-
102-01-2
N-phenylacetoacetamide

-
-
124095-27-8
(R)-3-hydroxy-N-phenylbutanamide

| Conditions | Yield |
|---|---|
| With <((R)-(1,1'-binaphthyl-2,2'-diyl)bis(diphenylphosphine))RuCl2>2NEt3; hydrogen In methanol at 60℃; under 22501.8 Torr; for 48h; | 99% |
| With hydrogen; {(RuCl[(R)-SYNPHOS])2(μ-Cl)3}[NH2Me2] In methanol at 50℃; under 7500.6 Torr; for 1h; | 92% |
-
-
110-52-1
1,4-dibromo-butane

-
-
102-01-2
N-phenylacetoacetamide

-
-
951000-13-8
1-acetylcyclopentanecarboxylic acid amide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 94% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; |

-
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With water; caesium carbonate; L-proline; copper(l) iodide In dimethyl sulfoxide at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane; water for 5h; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water at 20℃; for 0.333333h; Time; | 99% |
| With hydrogenchloride; sodium nitrite In water at 20℃; for 0.666667h; Green chemistry; | 99% |
| Stage #1: 4-methyl-2-nitroaniline With hydrogenchloride; C25H20N6O6S2; sodium nitrite In water at 10℃; for 1h; Stage #2: N-phenylacetoacetamide With sodium acetate; acetic acid In water at 25 - 90℃; for 2.5h; pH=6; | |
| Stage #1: 4-methyl-2-nitroaniline With hydrogenchloride; sodium nitrite In water at 10℃; for 1h; Stage #2: N-phenylacetoacetamide With sodium hydroxide; sodium acetate; acetic acid In water at 25 - 90℃; for 2.5h; pH=6; | |
| Stage #1: 4-methyl-2-nitroaniline With hydrogenchloride In water at 75 - 80℃; for 1h; Stage #2: With sodium nitrite In water at 0 - 5℃; for 0.75h; Stage #3: N-phenylacetoacetamide Further stages; |
-
-
102-01-2
N-phenylacetoacetamide

-
-
1439365-74-8
2,2-difluoro-3-oxo-N-phenylbutanamide

| Conditions | Yield |
|---|---|
| With 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2 2 2]octane bis(tetrafluoroborate); potassium carbonate In water at 20℃; for 5h; | 99% |
| With Selectfluor In water; acetonitrile at 20℃; for 16h; Schlenk technique; Sealed tube; chemoselective reaction; | 94% |

-
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In 1,4-dioxane at 20℃; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water at 20℃; for 0.333333h; Green chemistry; | 99% |
| Stage #1: 3,3'-dichlorobenzidine With hydrogenchloride; sodium nitrite In water at 0 - 5℃; for 0.5h; Large scale; Stage #2: N-phenylacetoacetamide With acetic acid; sodium hydroxide In water at 10 - 55℃; for 2.5h; pH=6.5 - 7; Reagent/catalyst; Large scale; | 580 kg |

-
-
102-01-2
N-phenylacetoacetamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-di-tert-butyl-4-(2-hydroxy-5-methylbenzylidene)cyclohexa-2,5-dienone; N-phenylacetoacetamide With 3-((3,5-bis(trifluoromethyl)phenyl)amino)-4-(((S)-(6-methoxyquinoline-4-yl))((1S,2S,4S,5R-5-vinylquinuclidine-2-yl)methyl)amino)cyclobutan-3-ene-1,2-dione In dichloromethane at 20℃; for 48h; Stage #2: With toluene-4-sulfonic acid In toluene at 110℃; for 1h; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-Chloro-2-nitroaniline With hydrogenchloride; sodium nitrite In water for 0.666667h; Stage #2: N-phenylacetoacetamide In water at 20℃; for 0.166667h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-nitro-aniline With hydrogenchloride; sodium nitrite In water at 20℃; for 0.333333h; Stage #2: N-phenylacetoacetamide In water at 20℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite In water at 20℃; for 0.75h; Time; Reagent/catalyst; | 99% |
| With hydrogenchloride; sodium nitrite In water at 20℃; for 1.5h; Temperature; Green chemistry; | 99% |
-
-
102-01-2
N-phenylacetoacetamide

-
-
100-46-9
benzylamine

-
-
59887-23-9
(Z)-3-(benzylamino)-N-phenylbut-2-enamide

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In water at 20℃; for 12h; | 98% |
| With iron(III) trifluoromethanesulfonate In neat (no solvent) at 20℃; | 68% |
-
-
7521-41-7
2-Aminonicotinaldehyde

-
-
102-01-2
N-phenylacetoacetamide

-
-
112697-61-7
2-methyl-N-phenyl-1,8-naphthyridine-3-carboxamide

| Conditions | Yield |
|---|---|
| With lithium chloride for 0.0833333h; Friedlaender condensation; microwave irradiation; | 98% |
| With toluene-4-sulfonic acid at 20℃; for 5h; Friedlander condensation; | 92% |
| With zirconyl chloride octahydrate In neat (no solvent, solid phase) at 70 - 80℃; for 0.15h; | 92% |
-
-
58663-95-9
5,7-dimethylpyrazolo<4,3-c><1,2,5>oxadiazin-3(5H)-one

-
-
102-01-2
N-phenylacetoacetamide

-
-
106538-03-8
1,3,6-Trimethyl-1H-pyrazolo[3,4-b]pyrazine-5-carboxylic acid phenylamide

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 0.5h; Ambient temperature; | 98% |
-
-
2919-20-2
1,1-di(p-tolyl)ethylene

-
-
102-01-2
N-phenylacetoacetamide

-
-
139050-83-2, 139050-84-3
6,6-bis(4-methylphenyl)-4-(phenylcarbamoyl)-3-methyl-1,2-dioxan-3-ol

| Conditions | Yield |
|---|---|
| With oxygen; manganese triacetate; acetic acid at 23℃; for 12h; | 98% |
-
-
102-01-2
N-phenylacetoacetamide

-
-
60-23-1
Cysteamine

-
-
122717-93-5
2-(2-Methyl-thiazolidin-2-yl)-N-phenyl-acetamide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 16h; Heating; | 98% |
| With toluene-4-sulfonic acid In benzene for 6h; Heating; | 98% |
-
-
156-57-0
2-mercaptoethylamine hydrochloride

-
-
102-01-2
N-phenylacetoacetamide

-
-
122717-93-5
2-(2-Methyl-thiazolidin-2-yl)-N-phenyl-acetamide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; triethylamine In benzene for 6h; Heating; | 98% |
-
-
59-88-1
phenylhydrazine hydrochloride

-
-
102-01-2
N-phenylacetoacetamide

-
-
22896-71-5
5-aminophenyl-3-methyl-1-phenyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| With pyridine; 2,4-{[3-[(CH2)5C8F17]-4-MeO-phenyl]}2-P2S2 2,4-disulfide In tetrahydrofuran at 55℃; for 14h; | 98% |
-
-
102-01-2
N-phenylacetoacetamide

-
-
106-96-7
propargyl bromide

-
-
1141891-26-0
2-acetyl-N-phenyl-2-(prop-2-ynyl)pent-4-ynamide

| Conditions | Yield |
|---|---|
| Stage #1: N-phenylacetoacetamide With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: propargyl bromide In N,N-dimethyl-formamide at 20℃; for 4h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide; acetone; toluene at 20℃; for 16h; | 93% |
| In tetrahydrofuran at 20℃; for 9h; |
-
-
103529-16-4
2-[(trimethylsilyl)ethynyl]aniline

-
-
102-01-2
N-phenylacetoacetamide

-
-
1220451-94-4
C18H16N2O

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethanol at 85℃; for 15h; | 98% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View

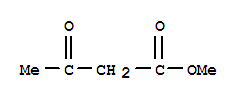













 Xn
Xn