Wuhan Fortuna Chemical Co.,Ltd
Unique advantages Guaranteed purity High quality & competitive price Quality control Fast feedback Prompt shipment Appearance:White to off-white powder Storage:cool dry place Package:25kg/drum Application:medicine raw material Transportatio
Cas:124937-51-5
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryXi'an Xszo Chem Co., Ltd.
1. Factory price and high quality must be guaranteed, base on 8 years of production and R&D experience2. Free samples will be provided,ensure specifications and quality are right for customer3. Customers will receive the most professional technical s
Cas:124937-51-5
Min.Order:1 Gram
FOB Price: $0.1
Type:Manufacturers
inquiryDayang Chem (Hangzhou) Co.,Ltd.
DayangChem exported this product to many countries and regions at best price in China. If you are looking for the product’s supplier in China, DayangChem is your best choice. Pls contact with us freely for getting detailed product specifica
Cas:124937-51-5
Min.Order:1 Kilogram
FOB Price: $2.0
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
Our advantages: 1, High quality with competitive price: 1) Standard:BP/USP/EP/Enterprise standard 2) All Purity≥99% 3) We are manufacturer and can provide high quality products with factory price. 2, Fast and safe delivery 1) Parcel can be sent
Cas:124937-51-5
Min.Order:1 Kilogram
FOB Price: $9.0 / 99.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:124937-51-5
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryLIDE PHARMACEUTICALS LIMITED
Advantage : LIDE PHARMACEUTICALS LTD. is a mid-small manufacturing-type enterprise, engaged in pharmaceutical intermediates of R&D, custom-made and production, and also involving trading chemicals for export. We have established the R&
Cas:124937-51-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryShanghai Upbio Tech Co.,Ltd
1.In No Less 10 years exporting experience. you can 100% received goods 2.Lower Price with higher quality 3,Free sample 4,We are sincerely responsible for the "product quality" and "After Service" Upbio is Specializ
Cas:124937-51-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryQingdao Beluga Import and Export Co., LTD
Tolterodine CAS:124937-51-5 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic intermediate
Cas:124937-51-5
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:124937-51-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHubei Jiutian Bio-medical Technology Co., Ltd
1,we produce and sell good chemicals around the world. 2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%. 3,our staff consists of hig
Cas:124937-51-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Hangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Shaanxi Cuicheng Biomedical Technology Co., Ltd.
Why Choose Us: 1. Factory direct sales, so we can provide the competitive price and high quality product base on 8 years of production and R&D experience. 2. It is available in stock for quick shipment.Products could be packaged according to cu
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:124937-51-5
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryTaiChem Taizhou Limited
Established in May 2015, TaiChem Ltd. is initially invested by a British research and development company and started by PhDs back from aboard. The company is registered in China Medical City (CMC), Taizhou, Jiangsu Province, and the production site
Ality Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providing h
Siwei Development Group Ltd.
Product name: Tolterodine CAS No.:124937-51-5 Molecule Formula:C22H31NO Molecule Weight:325.48 Purity: 99.0% Package: 25kg/drum Description:White crystalline powder Manufacture Standards:Enterprise Standard TESTING ITEMS
Cas:124937-51-5
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryJiangsu Qianyu Molecular Technology Co., LTD.
Our Advantages A. International Top level TechnologyOur company owned biomedicine experts are famous at home and abroad with rich experience in research and development in the field of efficient chiral functional molecules research and development an
Cas:124937-51-5
Min.Order:0
Negotiable
Type:Trading Company
inquiryXiamen Jenny Chemical Technology Co., Ltd.
GMP standard, high purity, competitive price, in stock 1. Quick Response: within 6 hours after receiving your email. 2. Quality Guarantee: All products are strictly tested by our QC, confirmed by QA, and approved by a third-party lab in China, USA,
Hunan chemfish Pharmaceutical co.,Ltd
Appearance:95%+ Package:R&D,Pilot run Transportation:per client require Port:Express ,Air, Sea
Suzhou Health Chemicals Co., Ltd.
High quality,stable supply chain.Appearance:white/off-white or light yellow Storage:Store in cool and dry place, keep away from strong light and heat. Package:aluminum bottle,glass bottle,PTFE bottle,cardboard drum Application:Active Pharmaceutical I
Golden Pharma Co., Limited
GOLDEN PHARMA CO.,LIMITED.is a professional pharmaceutical company,our team have more than 20years expereince in pharmaceutical production and sales. we are a professional technical enterprise specializing in the R & D, production,QA regulation
GIHI CHEMICALS CO.,LIMITED
Lower price, sample is available,SDS test documents are available,large stock in warehouseAppearance:White powder Storage:Sealed and preserved Package:200/Kilograms Application:Fine chemical intermediates, used as the main raw material for the synthe
Cas:124937-51-5
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquirySinoway Industrial Co., Ltd.
Why is SINOWAY:1) Specialized in pharmaceutical and healthcare industrial since 19872) ISO 9001:2015 & SGS audited supplier . 3) Accept various payment terms : T.T 30-60 days.4) We have warehouse in USA with quickly shipment .5) We can do different t
Cas:124937-51-5
Min.Order:0
Negotiable
Type:Trading Company
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea
Xi`an Eastling Biotech Co., Ltd.
high purity lowest priceAppearance:solid or liquid Storage:in sealed air resistant place Package:Foil bag; Drum; Plastic bottle Application:Pharma;Industry;Agricultural Transportation:by sea or air Port:Beijing or Guangzhou
Wuhan Circle Star Chem-medical Technology co.,Ltd.
1,we produce and sell good chemicals around the world.2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%.3,our staff consists of highly qualified in
Hangzhou Dawn Ray Pharmaceutical Co.,Ltd
Appearance: White or almost white crystalline powder Total impurity: ≤0.2% Assay :≥ 99.0% Appearance:White or almost white crystalline powder or crystals Storage:cool and dry Package:25kg/drum Application:A toadstool alkali receptor antagonis
Hebei Sankai Chemical Technology Co., Ltd
1. Product advantages? High purity, all above 98.5%, no impurities after dissolution? We will test each batch to ensure quality? OEM and private brand services designed for free? Various cap colors available? We can also provide MT1 peptide powder2.
Synthetic route
-
-
848768-06-9
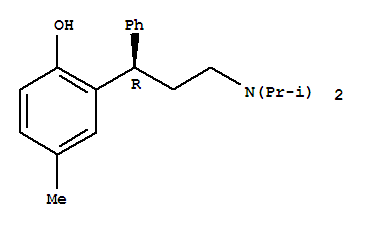
(R)-3-(2-(benzyloxy)-5-methylphenyl)-N,N-diisopropyl-3-phenylpropan-1-amine

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; for 24h; | 100% |
| With palladium on activated charcoal; hydrogen In methanol at 20℃; for 24h; | 100% |
| With hydrogen; palladium on activated charcoal In methanol at 20℃; under 760 Torr; for 12h; | 97% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; under 1125.11 Torr; for 24h; | 94% |

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In isopropyl alcohol at 50℃; for 22h; | 98% |
-
-
828933-86-4
(4R)-6-methyl-4-phenylchroman-2-ol

-
-
108-18-9
diisopropylamine

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In methanol at 20℃; for 72h; Inert atmosphere; | 93% |
| With palladium 10% on activated carbon; hydrogen In methanol at 50℃; under 2585.81 Torr; for 12h; Autoclave; | 93% |
| With hydrogen; palladium on activated charcoal In methanol under 2585.74 Torr; for 12h; Heating; | 91% |
-
-
1275593-51-5
(Z)-2-(3-(diisopropylamino)-1-phenylprop-1-enyl)-4-methylphenol

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With 1,2-bis [(2S,5S)-2,5-diphenylphospholano]ethane 1,5-cyclooctadiene rhodium(I) tetrafluoroborate; hydrogen; lithium tert-butoxide In toluene at 20℃; under 2585.81 Torr; for 2h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 89% |
-
-
518360-71-9
(R)-N,N-diisopropyl-3-(2-methoxy-5-methylphenyl)-3-phenylpropan-1-amine

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid | 87% |
| Stage #1: (R)-N,N-diisopropyl-3-(2-methoxy-5-methylphenyl)-3-phenylpropan-1-amine With boron tribromide In dichloromethane at -78 - 0℃; Stage #2: With methanol In dichloromethane at -78 - 20℃; optical yield given as %ee; |
-
-
906532-18-1
diisopropyl-(3-phenyl-3-p-toluyloxypropyl)amine

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Stage #1: diisopropyl-(3-phenyl-3-p-toluyloxypropyl)amine With sulfuric acid In water at 20℃; for 3h; Stage #2: With sodium hydroxide In water for 0.5h; pH=9 - 10; Product distribution / selectivity; | 82% |
| Stage #1: diisopropyl-(3-phenyl-3-p-toluyloxypropyl)amine at 20℃; for 3h; Stage #2: With sodium hydroxide In water for 0.5h; pH=9 - 10; Product distribution / selectivity; |
-
-
851789-55-4
C29H37NO3S

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 4h; Heating; | 82% |
-
-
106-44-5
p-cresol

-
-
906532-26-1
3-(N,N-diisopropylamino)-1-phenylpropan-1-ol

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Stage #1: p-cresol; 3-(N,N-diisopropylamino)-1-phenylpropan-1-ol With sulfuric acid In water at 40℃; for 3h; Stage #2: With sodium hydroxide In water for 0.5h; pH=9 - 10; Product distribution / selectivity; | 80% |
-
-
828933-86-4
(4R)-6-methyl-4-phenylchroman-2-ol

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid; diisopropylamine In methanol at 20℃; for 72h; | 48% |
-
-
215929-29-6
(R)-3-(2-Benzyloxy-5-methyl-phenyl)-N,N-diisopropyl-3-phenyl-propionamide

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; hydrogen; palladium on activated charcoal 1) ether, rt, overnight; 2) MeOH, 1 atm, rt, overnight; Yield given. Multistep reaction; | |
| Multi-step reaction with 2 steps 1: dimethylsulfide borane complex / tetrahydrofuran / 20 h / Inert atmosphere; Reflux 2: palladium 10% on activated carbon; hydrogen / methanol / 24 h / 20 °C / 1125.11 Torr View Scheme |

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Stage #1: 2-[3-[bis-(1-methylethyl)-amino]-1-phenylpropyl]-4-methylphenol tartrate In water; toluene pH=9.5; Stage #2: With formic acid; tartaric acid In isopropyl alcohol Purification / work up; |
-
-
16299-22-2
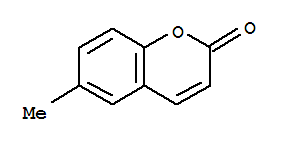
6-methyl-4-phenylchromen-2-one

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: R,R-chiraphos; NaOH; H2 / [Rh(COD)Cl]2 / methanol / 24 h / 50 °C / 9000.9 Torr 1.2: pTsOH / toluene / 4 h / Heating 1.3: DIBALH / toluene / -25 °C 2.1: H2 / Pd/C / methanol / 12 h / 48 °C / 3800.26 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: copper diacetate; diethoxymethylsilane; (R,R(Fc))-1-diphenylphosphino-2-(1-dicyclohexylphosphinoethyl)ferrocene / tetrahydrofuran; toluene; tert-butyl alcohol / 3 h / 20 °C / Inert atmosphere 2: sodium tris(acetoxy)borohydride; acetic acid / 1,2-dichloro-ethane / 72 h / 20 °C / Inert atmosphere View Scheme |
-
-
827007-19-2
(R)-6-methyl-4-phenyl-3,4-dihydrochromen-2-one

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / diisobutylaluminum hydride / toluene; hexane / 2.5 h / -25 - -20 °C 2: 91 percent / H2 / Pd/C / methanol / 12 h / 2585.74 Torr / Heating View Scheme | |
| Multi-step reaction with 4 steps 1.1: K2CO3 / 1 h / Heating 1.2: NaI / acetone / 36 h / 40 °C 1.3: 87 percent / LiAlH4 / tetrahydrofuran / 4 h / 0 °C 2.1: 83 percent / Et3N; DMAP / CH2Cl2 / 0 - 20 °C 3.1: 81 percent / K2CO3 / acetonitrile / 48 h / 60 °C 4.1: 97 percent / H2 / 10 percent Pd/C / methanol / 12 h / 20 °C / 760 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: diisobutylaluminium hydride / toluene; hexane / -20 - 25 °C / Schlenk technique; Inert atmosphere 2: hydrogen; palladium 10% on activated carbon / methanol / 12 h / 50 °C / 2585.81 Torr / Autoclave View Scheme |
-
-
92-48-8
6-methylcoumarin

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 88 percent / Rh(acac)(C2H4)2; (R)-Segphos / dioxane; H2O / 8 h / 60 °C 2: 95 percent / diisobutylaluminum hydride / toluene; hexane / 2.5 h / -25 - -20 °C 3: 91 percent / H2 / Pd/C / methanol / 12 h / 2585.74 Torr / Heating View Scheme |
-
-
98-80-6
phenylboronic acid

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 88 percent / Rh(acac)(C2H4)2; (R)-Segphos / dioxane; H2O / 8 h / 60 °C 2: 95 percent / diisobutylaluminum hydride / toluene; hexane / 2.5 h / -25 - -20 °C 3: 91 percent / H2 / Pd/C / methanol / 12 h / 2585.74 Torr / Heating View Scheme |
-
-
874651-73-7
(R)-3-(2-(benzyloxy)-5-methylphenyl)-3-phenylpropan-1-ol

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 83 percent / Et3N; DMAP / CH2Cl2 / 0 - 20 °C 2: 81 percent / K2CO3 / acetonitrile / 48 h / 60 °C 3: 97 percent / H2 / 10 percent Pd/C / methanol / 12 h / 20 °C / 760 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / ethyl acetate / Reflux 2: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 16 h / 20 °C 3: palladium on activated charcoal; hydrogen / methanol / 24 h / 20 °C View Scheme |
-
-
874651-74-8
(R)-3-[2-(benzyloxy)-5-methylphenyl]-3-phenylpropyl 4-nitrobenzenesulfonate

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 81 percent / K2CO3 / acetonitrile / 48 h / 60 °C 2: 97 percent / H2 / 10 percent Pd/C / methanol / 12 h / 20 °C / 760 Torr View Scheme |
-
-
2830-53-7
1-(benzyloxy)-2-bromo-4-methylbenzene

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) Mg, 2.) CuBr*Me2S, Me2S / 1) THF, reflux, 15 min; 2) THF, -50 deg C, 3 min; 3) THF, 10 deg C, 2 h 2: 90 percent / LiOH, H2O2 / tetrahydrofuran; H2O 3: 1.) SOCl2 / 1) benzene, 50 deg C, 50 min; 2) ether, rt, 1.5 h 4: 1.) LiAlH4, 2.) H2 / 2.) Pd/C / 1) ether, rt, overnight; 2) MeOH, 1 atm, rt, overnight View Scheme |
-
-
155835-37-3
(4R)-3-(3-phenyl-2-(E)-propenoyl)-4-phenyl-2-oxazolidinone

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) Mg, 2.) CuBr*Me2S, Me2S / 1) THF, reflux, 15 min; 2) THF, -50 deg C, 3 min; 3) THF, 10 deg C, 2 h 2: 90 percent / LiOH, H2O2 / tetrahydrofuran; H2O 3: 1.) SOCl2 / 1) benzene, 50 deg C, 50 min; 2) ether, rt, 1.5 h 4: 1.) LiAlH4, 2.) H2 / 2.) Pd/C / 1) ether, rt, overnight; 2) MeOH, 1 atm, rt, overnight View Scheme |
-
-
215929-28-5
(R)-3-(2-(benzyloxy)-5-methylphenyl)-3-phenylpropanoic acid

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) SOCl2 / 1) benzene, 50 deg C, 50 min; 2) ether, rt, 1.5 h 2: 1.) LiAlH4, 2.) H2 / 2.) Pd/C / 1) ether, rt, overnight; 2) MeOH, 1 atm, rt, overnight View Scheme | |
| Multi-step reaction with 3 steps 1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 24 h / 20 °C / Inert atmosphere 2: dimethylsulfide borane complex / tetrahydrofuran / 20 h / Inert atmosphere; Reflux 3: palladium 10% on activated carbon; hydrogen / methanol / 24 h / 20 °C / 1125.11 Torr View Scheme |
-
-
215929-27-4
(R)-3-[(R)-3-(2-benzyloxy-5-methylphenyl)-3-phenylpropionyl]-4-phenyloxazolidin-2-one

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / LiOH, H2O2 / tetrahydrofuran; H2O 2: 1.) SOCl2 / 1) benzene, 50 deg C, 50 min; 2) ether, rt, 1.5 h 3: 1.) LiAlH4, 2.) H2 / 2.) Pd/C / 1) ether, rt, overnight; 2) MeOH, 1 atm, rt, overnight View Scheme |

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In water; toluene |
-
-
124935-88-2
N,N-diisopropyl-3-(2-methoxy-5-methylphenyl)-3-phenylpropan-1-amine

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 0 - 5℃; |
-
-
897314-72-6
(+/-)-N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenyl-propanamide

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Stage #1: (+/-)-N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenyl-propanamide With lithium aluminium tetrahydride In diethyl ether for 96h; Heating / reflux; Stage #2: With acetic acid In diethyl ether pH=5; Product distribution / selectivity; |
-
-
40546-94-9
6-methyl-4-phenyl-3,4-dihydrocoumarin

-
-
108-18-9
diisopropylamine

-
A
-
851789-43-0
3-phenyl-3-(2'-hydroxy-5'-methyl)phenylpropanol-1

-
B
-
124937-51-5
(R)-(+)-tolterodine

-
C
-
209747-04-6
2-hydroxy-6-methyl-4-phenyldihydrobenzopyran

| Conditions | Yield |
|---|---|
| Stage #1: 6-methyl-4-phenyl-3,4-dihydrocoumarin With diisobutylaluminium hydride In toluene at -25℃; for 5h; Stage #2: With citric acid In water; ethyl acetate; toluene at 20℃; Stage #3: diisopropylamine With hydrogen; 5%-palladium/activated carbon In methanol at 48℃; under 3800.26 Torr; for 12h; |


-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: thionyl chloride / tetrahydrofuran / 12 h / 22 °C 2: acetonitrile / 8 h / 60 °C 3: hydrogen bromide / water; acetic acid / 4 h / 112 °C 4: 1,2-bis [(2S,5S)-2,5-diphenylphospholano]ethane 1,5-cyclooctadiene rhodium(I) tetrafluoroborate; hydrogen; lithium tert-butoxide / toluene / 2 h / 20 °C / 2585.81 Torr / Inert atmosphere View Scheme |

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: acetonitrile / 8 h / 60 °C 2: hydrogen bromide / water; acetic acid / 4 h / 112 °C 3: 1,2-bis [(2S,5S)-2,5-diphenylphospholano]ethane 1,5-cyclooctadiene rhodium(I) tetrafluoroborate; hydrogen; lithium tert-butoxide / toluene / 2 h / 20 °C / 2585.81 Torr / Inert atmosphere View Scheme |
-
-
4072-13-3
(2-methoxy-5-methylphenyl)phenylmethanone

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: tetrahydrofuran / 0 - 22 °C 2: thionyl chloride / tetrahydrofuran / 12 h / 22 °C 3: acetonitrile / 8 h / 60 °C 4: hydrogen bromide / water; acetic acid / 4 h / 112 °C 5: 1,2-bis [(2S,5S)-2,5-diphenylphospholano]ethane 1,5-cyclooctadiene rhodium(I) tetrafluoroborate; hydrogen; lithium tert-butoxide / toluene / 2 h / 20 °C / 2585.81 Torr / Inert atmosphere View Scheme |
-
-
613-84-3
2-hydroxy-5-methylbenzaldehyde

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: potassium carbonate 2.1: Inert atmosphere; Schlenk technique 3.1: triethylamine / toluene / 2 h / 150 °C / Inert atmosphere 4.1: sec.-butyllithium / diethyl ether; cyclohexane; hexane / 1 h / -78 °C / Inert atmosphere; Schlenk technique 4.2: 2 h / -78 °C / Inert atmosphere; Schlenk technique 4.3: 16.08 h / -78 - 20 °C / Inert atmosphere; Schlenk technique 5.1: tetrabutyl ammonium fluoride; water / toluene / 1.5 h / 20 °C 6.1: osmium(VIII) oxide; sodium periodate 7.1: sodium tris(acetoxy)borohydride / tetrahydrofuran / 18 h / 20 °C / Inert atmosphere 8.1: hydrogen bromide; acetic acid View Scheme | |
| Multi-step reaction with 4 steps 1.1: triphenylphosphine; lithium hydroxide monohydrate; lithium chloride / water / Reflux 2.1: calcium carbonate; palladium diacetate / methanol / 4 h / 60 °C 2.2: 20 °C 3.1: copper diacetate; diethoxymethylsilane; (R,R(Fc))-1-diphenylphosphino-2-(1-dicyclohexylphosphinoethyl)ferrocene / tetrahydrofuran; toluene; tert-butyl alcohol / 3 h / 20 °C / Inert atmosphere 4.1: sodium tris(acetoxy)borohydride; acetic acid / 1,2-dichloro-ethane / 72 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: triphenylphosphine; lithium hydroxide monohydrate; lithium chloride / water / Reflux 2: calcium carbonate; palladium diacetate / methanol / 60 °C 3: copper diacetate; diethoxymethylsilane; (R,R(Fc))-1-diphenylphosphino-2-(1-dicyclohexylphosphinoethyl)ferrocene / tetrahydrofuran; toluene; tert-butyl alcohol / 3 h / 20 °C / Inert atmosphere 4: sodium tris(acetoxy)borohydride; acetic acid / 1,2-dichloro-ethane / 72 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: toluene / 1.5 h / 80 °C 2.1: potassium carbonate / acetone / 20 °C 2.2: 3 h / Reflux 3.1: oxalyl dichloride; N,N-dimethyl-formamide / tetrahydrofuran / 0.5 h 4.1: n-butyllithium / tetrahydrofuran; hexane / 0.5 h / -78 °C 4.2: 0.75 h / -78 - 0 °C 5.1: palladium diacetate; [2,2]bipyridinyl / methanol; water / 16 h / 80 °C / Schlenk technique; Sealed tube 6.1: lithium borohydride / diethyl ether / 3 h / 0 - 20 °C / Inert atmosphere 7.1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / ethyl acetate / Reflux 8.1: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 16 h / 20 °C 9.1: palladium on activated charcoal; hydrogen / methanol / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: 18-crown-6 ether; potassium carbonate / acetone / 0.5 h / 20 °C 1.2: 3 h / Reflux 2.1: sodium hydride / mineral oil; tetrahydrofuran / 0.33 h / 0 °C / Inert atmosphere 2.2: 2 h / 0 - 20 °C / Inert atmosphere 3.1: potassium hydroxide; C47H55O7P; chlorobis(ethylene)rhodium(I) dimer / water; 1,4-dioxane / 24 h / 20 °C / Inert atmosphere 3.2: 3 h / Reflux 4.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 24 h / 20 °C / Inert atmosphere 5.1: dimethylsulfide borane complex / tetrahydrofuran / 20 h / Inert atmosphere; Reflux 6.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h / 20 °C / 1125.11 Torr View Scheme |
-
-
79-30-1
isobutyryl chloride

-
-
124937-51-5
(R)-(+)-tolterodine

-
-
895137-81-2
2-[(1R)-3-(diisopropylamino)-1-phenylpropyl]-4-methylphenyl 2-methylpropanoate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 1h; Inert atmosphere; | 96% |
| With sodium hydroxide In tetrahydrofuran; water at 20℃; Inert atmosphere; | 96% |
| Stage #1: isobutyryl chloride; (R)-(+)-tolterodine In dichloromethane at -10 - 0℃; Stage #2: With sodium hydrogencarbonate In dichloromethane; water for 0.25h; |

| Conditions | Yield |
|---|---|
| In acetone at 25℃; Inert atmosphere; | 92.3% |
| In acetone at 25℃; | 92.3% |
-
-
98-11-3
benzenesulfonic acid

-
-
124937-51-5
(R)-(+)-tolterodine

-
-
1182264-73-8
tolterodine benzene sulfonate

| Conditions | Yield |
|---|---|
| In acetone at 25℃; Inert atmosphere; | 89.9% |
| In acetone at 25℃; | 89.9% |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 4h; Temperature; | 87.27% |
-
-
124937-51-5
(R)-(+)-tolterodine

-
-
86-48-6
1-hydroxy-2-naphthoic acid

-
-
615255-99-7
tolterodine 1-hydroxy-2-naphthate

| Conditions | Yield |
|---|---|
| In acetone at 25℃; Inert atmosphere; | 84.1% |
| In acetone at 25℃; | 84.1% |
-
-
104-15-4
toluene-4-sulfonic acid

-
-
124937-51-5
(R)-(+)-tolterodine

-
-
1182264-77-2
tolterodine p-toluene sulfonate

| Conditions | Yield |
|---|---|
| In acetone at 25℃; Inert atmosphere; | 83% |
| In acetone at 25℃; | 83% |
-
-
124937-51-5
(R)-(+)-tolterodine

-
-
98-79-3
L-Pyroglutamic acid

-
-
1182264-79-4
tolterodine L-pyroglutamate

| Conditions | Yield |
|---|---|
| In acetone at 25℃; | 82.3% |
| In acetone at 25℃; for 1.16667h; | 82.3% |
-
-
81-04-9
naphthalene-1,5-disulfonate

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| In acetone at 25℃; for 1.16667h; | 78.4% |
| In acetone at 25℃; for 1.16667h; | 78.4% |

| Conditions | Yield |
|---|---|
| In acetone at 25℃; for 1.16667h; | 74.3% |

| Conditions | Yield |
|---|---|
| In acetone at 25℃; for 1.16667h; | 74.3% |

| Conditions | Yield |
|---|---|
| In acetone at 25℃; for 1.16667h; | 73.1% |
| In acetone at 25℃; for 1.16667h; | 73.1% |
-
-
79-14-1
glycolic Acid

-
-
124937-51-5
(R)-(+)-tolterodine

-
-
1182264-86-3
(R)-2-(3-(diisopropylamino)-1-phenylpropyl)-4-methylphenol 2-hydroxyacetate

| Conditions | Yield |
|---|---|
| In acetone at 25℃; for 1.16667h; | 69.2% |
| In acetone at 25℃; for 1.16667h; | 69.2% |
-
-
490-79-9
2,5-dihydroxybenzoic acid.

-
-
124937-51-5
(R)-(+)-tolterodine

-
-
1182264-71-6
tolterodine gentisate

| Conditions | Yield |
|---|---|
| In acetone at 25℃; Inert atmosphere; | 42.5% |
| In acetone at 25℃; | 42.5% |
-
-
97950-17-9
(2Z,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoic acid

-
-
124937-51-5
(R)-(+)-tolterodine

| Conditions | Yield |
|---|---|
| In acetone at 25℃; | 22.4% |

| Conditions | Yield |
|---|---|
| In ethanol; acetone at 25℃; | 22.4% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View