-
Name
BETA-RHODINOL
- EINECS 231-415-7
- CAS No. 7540-51-4
- Article Data61
- CAS DataBase
- Density 0.845 g/cm3
- Solubility SLIGHTLY SOLUBLE
- Melting Point 77-83oC(lit.)
- Formula C10H20O
- Boiling Point 224.5 °C at 760 mmHg
- Molecular Weight 156.268
- Flash Point 98.3 °C
- Transport Information
- Appearance Clear colorless liquid
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 6-Octen-1-ol,3,7-dimethyl-, (-)- (8CI);6-Octen-1-ol, 3,7-dimethyl-, (S)-;(-)-(S)-Citronellol;(-)-Citronellol;(-)-b-Citronellol;(S)-(-)-Citronellol;(S)-(-)-b-Citronellol;(S)-3,7-Dimethyl-6-octen-1-ol;(S)-Citronellol;(S)-b-Citronellol;L-Citronellol;
- PSA 20.23000
- LogP 2.75130
Synthetic route

| Conditions | Yield |
|---|---|
| With bis(norbornadiene)rhodium(l)tetrafluoroborate; (RC,SFc,SP)-1-[2-(1-N,N-dimethylaminoethyl)ferrocen-1-yl]phenylphosphino-1'-dicyclohexylphosphinoferrocene; hydrogen In dichloromethane at 20℃; under 19001.3 Torr; for 20h; Inert atmosphere; Autoclave; enantioselective reaction; | 99% |
| With [RuCl(p-cymene)((R)-Tol-BINAP)]Cl; potassium hydroxide In isopropyl alcohol for 2h; Inert atmosphere; Reflux; | 70% |
| Multi-step reaction with 2 steps 1: in Ficus retusa Linn. 2: in Ficus retusa Linn. nach Injektion View Scheme |

| Conditions | Yield |
|---|---|
| With dichloro(1,5-cyclooctadiene)ruthenium(II); (R)-(+)-2,2',6,6'-tetramethoxy-4,4'-bis(diphenylphosphino)-3,3'-bipyridine; niobium pentachloride; HSiPh3 In toluene at 40℃; for 3h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Glovebox; Sealed tube; Inert atmosphere; enantioselective reaction; | 98.59% |
| With (η4-cyclooctadiene)((R)-2,2'-bis(diphenylphosphanyl)-1,1'-binaphthyl)rhodium(I) tetrfluoroborate; hydrogen In methanol at 60℃; under 33753.4 Torr; for 5h; Autoclave; enantioselective reaction; | 88% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With indium; water; ammonium chloride In methanol for 1h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether | 96% |
| With lithium aluminium tetrahydride In diethyl ether | 94% |
| With sodium tetrahydroborate In ethanol at 0℃; | 93% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With indium; water; ammonium chloride In methanol for 0.5h; Heating; | 95% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With sodium perborate; water In tetrahydrofuran at 20℃; for 4h; enantioselective reaction; | 95% |
-
-
93041-00-0
2,2-dimethyl-propionic acid (3S)-3,7-dimethyl-oct-6-enyl ester

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol Ambient temperature; | 94% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-8-(allyloxy)-2,6-dimethyloct-2-ene With C12H37NiP4(1+)*C2F6NO4S2(1-) In tetrahydrofuran at 20℃; for 0.5h; Glovebox; Schlenk technique; Inert atmosphere; Stage #2: With toluene-4-sulfonic acid In tetrahydrofuran for 1.5h; Glovebox; Schlenk technique; Reflux; Inert atmosphere; | 94% |
-
-
89156-40-1
(S)-3,7-Dimethyl-oct-6-enoic acid (1S,2R,3S,4R)-3-(2,2-dimethyl-propoxy)-4,7,7-trimethyl-bicyclo[2.2.1]hept-2-yl ester

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
85695-96-1
(1R,2S,3R,4S)-2-(2,2-dimethylpropoxy)-1,7,7-trimethylbicyclo<2.2.1>heptan-3-ol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride | A 88% B 81% |
-
-
84237-05-8
(S)-((3,7-dimethyloct-6-enyloxy)methyl)benzene

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With ammonia; lithium In tetrahydrofuran at -30℃; for 0.666667h; | 82% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With (PhSO2)2; water In dichloromethane at 80℃; | 82% |
-
-
5949-05-3
(S)-Citronellal

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
2111-53-7
(S)-(-)-citronellic acid

| Conditions | Yield |
|---|---|
| With Fusarium concentricum In dimethyl sulfoxide at 28 - 30℃; for 120h; Reagent/catalyst; Microbiological reaction; | A 76% B 5% |
| With Fusarium fujikuroi In dimethyl sulfoxide at 28 - 30℃; for 120h; Microbiological reaction; | A 36% B 43% |
| With endosperms of Triticum aestivum L. cv Dariel wheat seeds In water at 27℃; Reagent/catalyst; Concentration; Darkness; Enzymatic reaction; enantioselective reaction; |

| Conditions | Yield |
|---|---|
| With 1,1′-binaphthalene-2,2′-diylbis[bis(4-methylphenyl)phosphine]; potassium hydroxide at 100℃; for 2h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 70% |
| With hydrogen; BINAP-Ru(II) dicarboxylate In methanol at 18 - 20℃; Yield given; | |
| With hydrogen; BINAP-(Ru(II) dicarboxylate In methanol at 18 - 20℃; Product distribution; examination of enantioselectivity; various complexes; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 25℃; for 3h; Inert atmosphere; | 69% |
-
-
5949-05-3
(S)-Citronellal

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
19956-48-0
(1S,3R,4S)-p-menthane-3,8-diol


-
D
(−)-neo-isopulegol -
122517-60-6
(−)-neo-isopulegol

| Conditions | Yield |
|---|---|
| With Penicillium paxilli In dimethyl sulfoxide at 28 - 30℃; for 120h; Microbiological reaction; | A 10% B 50% C 20% D 5% E 2% |


-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With potassium chloride; water In acetone | 48% |
-
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

-
A
-
2385-77-5
(R)-Citronellal

-
B
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With baker's yeast | A 21% B 33% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With potassium (4-methylphenyl)trifluoroborate In dimethyl sulfoxide at 37℃; for 24h; Inert atmosphere; | 18% |

| Conditions | Yield |
|---|---|
| in Ficus retusa Linn. nach Injektion; |

| Conditions | Yield |
|---|---|
| With hydrogen; <(+)-CyBINAP>-RhN In benzene under 15200 Torr; Ambient temperature; Yield given. Yields of byproduct given; | |
| With hydrogen; ClO4 In benzene under 7600 Torr; for 8h; Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With hydrogen; Ru2Cl4-((S)-2,2'-bis[di(p-tolyl)phosphino]-1,1'-binaphthyl)2NEt3 In methanol at 19.9℃; under 1807.6 Torr; Product distribution; different pressure and temperature; | |
| With 1,2-bis(2,5-diisopropylphospholano)benzene; potassium hydroxide at 100℃; for 24h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; |
-
-
106-24-1
Geraniol

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
1117-61-9
(3R)-citronellol

-
C
-
1117-60-8
(R)-3,7-dimethyloctan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; {RuI((R)-2,2'-bis(diphenylphospino)-1,1'-binaphthyl)(p-cymene)}I In methanol; water at 20℃; under 76000 Torr; for 8h; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With <<(-)-2,2'-bis(diphenylphosphino)-4,4',6,6'-tetramethyl-3,3'-bibenzothiophene>Ru(p-cumene)I>I; hydrogen In methanol at 25℃; under 7355.08 Torr; for 88h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With ((S)-(6,6'-dimethylbiphenyl-2,2'-diyl)bis(diphenylphosphine))Ru(O2CCF3)2; hydrogen In methanol at 20 - 25℃; under 45003.6 Torr; for 1h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With hydrogen; <(+)-BINAP>RhN In benzene under 22800 Torr; Ambient temperature; Yield given. Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With lithium triethylborohydride In tetrahydrofuran Ambient temperature; Yield given; | |
| With lithium triethylborohydride In tetrahydrofuran Ambient temperature; Yields of byproduct given; |
-
-
67392-54-5
N,N-diethyl-N-{1-[(3S),7-dimethylocta-1,6-dienyl]}amine

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With oxonium; hydrogen |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate; 2-methyl-but-2-ene; boron trifluoride; dihydrogen peroxide 1.) THF, room temp., 3 h.; 2.) 50 deg C, 1.5 h; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With Ru2Cl4-((S)-2,2'-bis[di(p-tolyl)phosphino]-1,1'-binaphthyl)2NEt3; hydrogen In methanol at 19.9℃; under 3750.3 Torr; Title compound not separated from byproducts; | |
| With hydrogen; Ru2Cl4-((S)-2,2'-bis[di(p-tolyl)phosphino]-1,1'-binaphthyl)2NEt3 In methanol at 19.9℃; under 1807.6 Torr; Product distribution; other pressure and temperature; | |
| With 1,1′-binaphthalene-2,2′-diylbis[bis(4-methylphenyl)phosphine]; potassium hydroxide at 100℃; for 6h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; |
-
-
67601-05-2
(3S)-3,7-dimethyl-6-octen-1-yl acetate

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: (3S)-3,7-dimethyl-6-octen-1-yl acetate With sodium hydroxide In ethanol at 90℃; for 3h; Alkaline hydrolysis; Stage #2: With Pichia kluyveri IFO 1165 at 30℃; for 48h; |
-
-
108-05-4
vinyl acetate

-
-
106-22-9
Citronellol

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
1117-61-9
(3R)-citronellol

-
C
-
20425-54-1
(R)-1-citronellyl acetate

-
D
-
67601-05-2
(3S)-3,7-dimethyl-6-octen-1-yl acetate

| Conditions | Yield |
|---|---|
| With porcine pancreatic lipase In hexane for 24h; Title compound not separated from byproducts; | |
| With porcine pancreatic lipase In hexane for 48h; Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With bis(norbornadiene)rhodium(l)tetrafluoroborate; (RC,SFc,SP)-1-[2-(1-N,N-dimethylaminoethyl)ferrocen-1-yl]phenylphosphino-1'-dicyclohexylphosphinoferrocene; hydrogen In dichloromethane at 20℃; under 19001.3 Torr; for 20h; Inert atmosphere; Autoclave; enantioselective reaction; | 99% |
| With [RuCl(p-cymene)((R)-Tol-BINAP)]Cl; potassium hydroxide In isopropyl alcohol for 2h; Inert atmosphere; Reflux; | 70% |
| Multi-step reaction with 2 steps 1: in Ficus retusa Linn. 2: in Ficus retusa Linn. nach Injektion View Scheme |

| Conditions | Yield |
|---|---|
| With dichloro(1,5-cyclooctadiene)ruthenium(II); (R)-(+)-2,2',6,6'-tetramethoxy-4,4'-bis(diphenylphosphino)-3,3'-bipyridine; niobium pentachloride; HSiPh3 In toluene at 40℃; for 3h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Glovebox; Sealed tube; Inert atmosphere; enantioselective reaction; | 98.59% |
| With (η4-cyclooctadiene)((R)-2,2'-bis(diphenylphosphanyl)-1,1'-binaphthyl)rhodium(I) tetrfluoroborate; hydrogen In methanol at 60℃; under 33753.4 Torr; for 5h; Autoclave; enantioselective reaction; | 88% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With indium; water; ammonium chloride In methanol for 1h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether | 96% |
| With lithium aluminium tetrahydride In diethyl ether | 94% |
| With sodium tetrahydroborate In ethanol at 0℃; | 93% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With indium; water; ammonium chloride In methanol for 0.5h; Heating; | 95% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With sodium perborate; water In tetrahydrofuran at 20℃; for 4h; enantioselective reaction; | 95% |
-
-
93041-00-0
2,2-dimethyl-propionic acid (3S)-3,7-dimethyl-oct-6-enyl ester

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol Ambient temperature; | 94% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-8-(allyloxy)-2,6-dimethyloct-2-ene With C12H37NiP4(1+)*C2F6NO4S2(1-) In tetrahydrofuran at 20℃; for 0.5h; Glovebox; Schlenk technique; Inert atmosphere; Stage #2: With toluene-4-sulfonic acid In tetrahydrofuran for 1.5h; Glovebox; Schlenk technique; Reflux; Inert atmosphere; | 94% |
-
-
89156-40-1
(S)-3,7-Dimethyl-oct-6-enoic acid (1S,2R,3S,4R)-3-(2,2-dimethyl-propoxy)-4,7,7-trimethyl-bicyclo[2.2.1]hept-2-yl ester

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
85695-96-1
(1R,2S,3R,4S)-2-(2,2-dimethylpropoxy)-1,7,7-trimethylbicyclo<2.2.1>heptan-3-ol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride | A 88% B 81% |
-
-
84237-05-8
(S)-((3,7-dimethyloct-6-enyloxy)methyl)benzene

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With ammonia; lithium In tetrahydrofuran at -30℃; for 0.666667h; | 82% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With (PhSO2)2; water In dichloromethane at 80℃; | 82% |
-
-
5949-05-3
(S)-Citronellal

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
2111-53-7
(S)-(-)-citronellic acid

| Conditions | Yield |
|---|---|
| With Fusarium concentricum In dimethyl sulfoxide at 28 - 30℃; for 120h; Reagent/catalyst; Microbiological reaction; | A 76% B 5% |
| With Fusarium fujikuroi In dimethyl sulfoxide at 28 - 30℃; for 120h; Microbiological reaction; | A 36% B 43% |
| With endosperms of Triticum aestivum L. cv Dariel wheat seeds In water at 27℃; Reagent/catalyst; Concentration; Darkness; Enzymatic reaction; enantioselective reaction; |

| Conditions | Yield |
|---|---|
| With 1,1′-binaphthalene-2,2′-diylbis[bis(4-methylphenyl)phosphine]; potassium hydroxide at 100℃; for 2h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 70% |
| With hydrogen; BINAP-Ru(II) dicarboxylate In methanol at 18 - 20℃; Yield given; | |
| With hydrogen; BINAP-(Ru(II) dicarboxylate In methanol at 18 - 20℃; Product distribution; examination of enantioselectivity; various complexes; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 25℃; for 3h; Inert atmosphere; | 69% |
-
-
5949-05-3
(S)-Citronellal

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
19956-48-0
(1S,3R,4S)-p-menthane-3,8-diol


-
D
-
122517-60-6
(−)-neo-isopulegol

-
E
-
104870-56-6
(+)-isopulegol

| Conditions | Yield |
|---|---|
| With Penicillium paxilli In dimethyl sulfoxide at 28 - 30℃; for 120h; Microbiological reaction; | A 10% B 50% C 20% D 5% E 2% |


-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With potassium chloride; water In acetone | 48% |
-
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

-
A
-
2385-77-5
(R)-Citronellal

-
B
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With baker's yeast | A 21% B 33% |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With potassium (4-methylphenyl)trifluoroborate In dimethyl sulfoxide at 37℃; for 24h; Inert atmosphere; | 18% |

| Conditions | Yield |
|---|---|
| in Ficus retusa Linn. nach Injektion; |

| Conditions | Yield |
|---|---|
| With hydrogen; <(+)-CyBINAP>-RhN In benzene under 15200 Torr; Ambient temperature; Yield given. Yields of byproduct given; | |
| With hydrogen; ClO4 In benzene under 7600 Torr; for 8h; Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With hydrogen; Ru2Cl4-((S)-2,2'-bis[di(p-tolyl)phosphino]-1,1'-binaphthyl)2NEt3 In methanol at 19.9℃; under 1807.6 Torr; Product distribution; different pressure and temperature; | |
| With 1,2-bis(2,5-diisopropylphospholano)benzene; potassium hydroxide at 100℃; for 24h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; |
-
-
106-24-1
Geraniol

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
1117-61-9
(3R)-citronellol

-
C
-
1117-60-8
(R)-3,7-dimethyloctan-1-ol

| Conditions | Yield |
|---|---|
| With hydrogen; {RuI((R)-2,2'-bis(diphenylphospino)-1,1'-binaphthyl)(p-cymene)}I In methanol; water at 20℃; under 76000 Torr; for 8h; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With <<(-)-2,2'-bis(diphenylphosphino)-4,4',6,6'-tetramethyl-3,3'-bibenzothiophene>Ru(p-cumene)I>I; hydrogen In methanol at 25℃; under 7355.08 Torr; for 88h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With ((S)-(6,6'-dimethylbiphenyl-2,2'-diyl)bis(diphenylphosphine))Ru(O2CCF3)2; hydrogen In methanol at 20 - 25℃; under 45003.6 Torr; for 1h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With hydrogen; <(+)-BINAP>RhN In benzene under 22800 Torr; Ambient temperature; Yield given. Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With lithium triethylborohydride In tetrahydrofuran Ambient temperature; Yield given; | |
| With lithium triethylborohydride In tetrahydrofuran Ambient temperature; Yields of byproduct given; |
-
-
67392-54-5
N,N-diethyl-N-{1-[(3S),7-dimethylocta-1,6-dienyl]}amine

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| With oxonium; hydrogen |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate; 2-methyl-but-2-ene; boron trifluoride; dihydrogen peroxide 1.) THF, room temp., 3 h.; 2.) 50 deg C, 1.5 h; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With Ru2Cl4-((S)-2,2'-bis[di(p-tolyl)phosphino]-1,1'-binaphthyl)2NEt3; hydrogen In methanol at 19.9℃; under 3750.3 Torr; Title compound not separated from byproducts; | |
| With hydrogen; Ru2Cl4-((S)-2,2'-bis[di(p-tolyl)phosphino]-1,1'-binaphthyl)2NEt3 In methanol at 19.9℃; under 1807.6 Torr; Product distribution; other pressure and temperature; | |
| With 1,1′-binaphthalene-2,2′-diylbis[bis(4-methylphenyl)phosphine]; potassium hydroxide at 100℃; for 6h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; |
-
-
67601-05-2
(3S)-3,7-dimethyl-6-octen-1-yl acetate

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: (3S)-3,7-dimethyl-6-octen-1-yl acetate With sodium hydroxide In ethanol at 90℃; for 3h; Alkaline hydrolysis; Stage #2: With Pichia kluyveri IFO 1165 at 30℃; for 48h; |
-
-
108-05-4
vinyl acetate

-
-
106-22-9
Citronellol

-
A
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
B
-
1117-61-9
(3R)-citronellol

-
C
-
20425-54-1
(R)-1-citronellyl acetate

-
D
-
67601-05-2
(3S)-3,7-dimethyl-6-octen-1-yl acetate

| Conditions | Yield |
|---|---|
| With porcine pancreatic lipase In hexane for 24h; Title compound not separated from byproducts; | |
| With porcine pancreatic lipase In hexane for 48h; Title compound not separated from byproducts; |

-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
-
68680-98-8
S-(-)-dihydrocitronellol

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen | 100% |
| With palladium on activated charcoal; hydrogen In methanol; ethyl acetate at 25℃; for 18h; Inert atmosphere; | 98% |
| With palladium 10% on activated carbon; hydrogen In ethyl acetate at 20℃; for 0.25h; Inert atmosphere; | 96% |
-
-
7540-51-4
(S)-3,7-dimethyl-6-octen-1-ol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
41144-01-8, 67214-54-4, 93303-23-2, 116661-43-9
(S)-3,7-dimethyl-6-octenyl tosylate

| Conditions | Yield |
|---|---|
| With pyridine at 0℃; for 12h; Tosylation; | 100% |
| With pyridine at 0℃; for 72h; | 100% |
| With pyridine | 100% |
(-)-beta-Citronellol Chemical Properties
Molecular structure of 6-Octen-1-ol, 3,7-dimethyl-, (3S)- (CAS NO.7540-51-4) is:
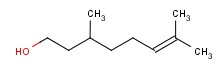
Product Name: 6-Octen-1-ol, 3,7-dimethyl-, (3S)-
CAS Registry Number: 7540-51-4
IUPAC Name: 3,7-dimethyloct-6-en-1-ol
Molecular Weight: 156.2652 [g/mol]
Molecular Formula: C10H20O
XLogP3-AA: 3.2
H-Bond Donor: 1
H-Bond Acceptor: 1
EINECS: 231-415-7
Surface Tension: 28.5 dyne/cm
Density: 0.845 g/cm3
Flash Point: 98.3 °C
Enthalpy of Vaporization: 53.6 kJ/mol
Boiling Point: 224.5 °C at 760 mmHg
Vapour Pressure: 0.0183 mmHg at 25°C
Vapor pressure: ~0.02 mm Hg ( 25 °C)
Refractive index: n20/D 1.456
Storage temp.: 2-8°C
Product Categories: All Aliphatics ;Acyclic Monoterpenes ;Biochemistry ;Terpenes ;Aliphatics ;Chiral Reagents
(-)-beta-Citronellol Safety Profile
Safty information about 6-Octen-1-ol, 3,7-dimethyl-, (3S)- (CAS NO.7540-51-4) is:
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 2
F: 8-10
(-)-beta-Citronellol Specification
6-Octen-1-ol, 3,7-dimethyl-, (3S)- , its cas register number is 7540-51-4. It also can be called AI3-09552 ; EINECS 231-415-7 ; FEMA No. 2309 ; beta-Citronellol ; l-Citronellol ; (-)-3,7-Dimethyloct-6-en-1-ol ; 6-Octen-1-ol, 3,7-dimethyl-, (S)- .It is a clear colorless liquid.
Related Products
- (-)-beta-Citronellol
- 754-05-2
- 75410-53-6
- 754-10-9
- 7541-17-5
- 754-12-1
- 75414-00-5
- 7541-49-3
- 754153-28-1
- 75415-78-0
- 754159-68-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View