-
Name
(2-Bromoethyl)benzene
- EINECS 203-130-8
- CAS No. 103-63-9
- Article Data128
- CAS DataBase
- Density 1.366 g/cm3
- Solubility Miscible with ether, benzene, insoluble in water
- Melting Point -56 °C
- Formula C8H9Br
- Boiling Point 220.5 °C at 760 mmHg
- Molecular Weight 185.063
- Flash Point 89.4 °C
- Transport Information
- Appearance Colourless to yellow liquid
- Safety 26-37/39
- Risk Codes 22-36-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2-Bromoethyl benzene;1-Bromo-2-phenylethane;1-Phenyl-2-bromoethane;2-Bromo-1-phenylethane;2-Phenethyl bromide;2-Phenyl-1-bromoethane;2-Phenylbromoethane;2-Phenylethylbromide;NSC 33926;Phenethyl bromide;Phenylethyl bromide;b-Bromoethylbenzene;b-Phenethyl bromide;b-Phenylethyl bromide;
- PSA 0.00000
- LogP 2.62400
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen bromide; Aliquat 336 In chlorobenzene at 85℃; without cat.; | 98.7% |
| With 1,2-dibromo-1,1,2,2-tetrachloroethane; triphenylphosphine In dichloromethane at 20℃; for 0.15h; Appel Halogenation; | 97% |
| With Silphos; bromine In acetonitrile for 0.416667h; Heating; | 96% |
-
-
20020-27-3
2-phenylethyl mesylate

-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With 1-n-butyl-3-methylimidazolim bromide at 50℃; for 1h; Inert atmosphere; Green chemistry; | 97% |
| With lithium bromide In tetrahydrofuran Inert atmosphere; Reflux; | 94% |

| Conditions | Yield |
|---|---|
| With 2,4,4,6-Tetrabromo-2,5-cyclohexadien-1-one; silica gel for 4h; UV-irradiation; | 94% |
| With dihydrogen peroxide; bromine In dichloromethane; water for 4h; Reagent/catalyst; Reflux; | 92% |

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 0℃; for 0.0833333h; | 91% |
| With aluminum tri-bromide In chlorobenzene at 20℃; for 1h; |
-
-
54673-12-0
(2-(methoxymethoxy)ethyl)benzene

-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; tetrabutylammomium bromide at 130 - 142℃; for 0.0333333h; Microwave irradiation; Ionic liquid; chemoselective reaction; | 90% |
| With tetrabutylammomium bromide; 1-(n-butyl)-3-methylimidazolium tetrachloroindate at 135 - 140℃; for 0.0666667h; Microwave irradiation; Neat (no solvent); chemoselective reaction; | 83% |
-
-
76192-15-9
4-(4-Chloro-phenyl)-1-phenethyl-2,3,5,6-tetraphenyl-pyridinium; bromide

-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With 2,4,6-triphenylpyridine at 180 - 220℃; under 0.5 - 1.5 Torr; | 75% |
-
-
165904-22-3
4,4,5,5-tetramethyl-2-phenethyl-1,3,2-dioxaborolane

-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; 2-methoxybenzo[d][1,3,2]dioxaborole; benzenesulfonyl bromide In methanol; dichloromethane; benzene at 70℃; for 16h; Inert atmosphere; | 75% |
-
-
60-12-8
2-phenylethanol

-
-
603-35-0
triphenylphosphine

-
A
-
103-63-9
1-phenyl-2-bromoethane

-
B
-
791-28-6
Triphenylphosphine oxide

| Conditions | Yield |
|---|---|
| With carbon tetrabromide In 1,2-dichloro-ethane | A 72% B n/a |


| Conditions | Yield |
|---|---|
| With lithium bromide In N,N-dimethyl-formamide at 120℃; Inert atmosphere; | A 17% B 72% |

-
-
106-95-6
allyl bromide

-
A
-
1227781-92-1
3,5-diphenyl-4-(2-propen-1-yl)isoxazole

-
B
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; palladium diacetate In N,N-dimethyl-formamide at 80℃; for 1h; | A 68% B 65 %Spectr. |

-
-
159690-31-0
1-p-Toluolsulfonyl-1-phenethyl-hydrazin

-
A
-
1950-69-2
toluene-p-sulfonyl bromide

-
B
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrahydrofuran for 16h; Ambient temperature; Irradiation; | A n/a B 67% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-phenylethanol With N,N-dimethylthiourea In dichloromethane at 20℃; Stage #2: With N-Bromosuccinimide In dichloromethane at 20℃; for 2h; | A n/a B 64% |
-
-
71017-60-2
1-Phenethyl-2,3,4,5,6-pentaphenyl-pyridinium; bromide

-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With 2,4,6-triphenylpyridine at 180 - 220℃; under 0.5 - 2 Torr; | 60% |
-
-
108-88-3
toluene

-
-
74-95-3
1,2-dibromomethane

-
A
-
1081-75-0
1,3-diphenylpropane

-
B
-
103-63-9
1-phenyl-2-bromoethane

-
C
-
103-29-7
1,1'-(1,2-ethanediyl)bisbenzene

| Conditions | Yield |
|---|---|
| Stage #1: toluene With n-butyllithium In tetrahydrofuran; hexane at 20℃; for 3h; Inert atmosphere; Schlenk technique; Stage #2: 1,2-dibromomethane In tetrahydrofuran; hexane at 20℃; Inert atmosphere; Schlenk technique; | A 1% B n/a C n/a |

-
-
292638-84-7
styrene

-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With dilauryl peroxide; hydrogen bromide; pentane | |
| With ethylbenzene; hydrogen bromide; dibenzoyl peroxide at 95℃; | |
| With hydrogen bromide; nickel; Petroleum ether |
-
-
506-68-3
bromocyane

-
-
5300-21-0
N,N-diethylphenethylamine

-
A
-
74-96-4
ethyl bromide

-
B
-
860767-75-5
ethyl-phenethyl-carbamonitrile

-
C
-
103-63-9
1-phenyl-2-bromoethane

-
-
40515-89-7
2-phenethoxybenzene

-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 120℃; | |
| With water; boron tribromide In dichloromethane at 20℃; for 0.5h; Inert atmosphere; | 98 %Chromat. |
-
-
53800-00-3
5,8-Dibrom-1,3-cyclooctadien

-
A
-
585-71-7, 38661-81-3
(1-bromoethyl)benzne

-
B
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| under 760 Torr; Erhitzen auf Siedetemperatur; |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; 1,1,3,3-Tetramethyldisiloxane; lithium bromide 1.) dry acetonitrile, 5-10 deg C, 2-3 min, 2.) reflux, 15 min; Yield given. Multistep reaction; | |
| With chloro-trimethyl-silane; 1,1,3,3-Tetramethyldisiloxane; lithium bromide 1.) trifluoroacetic acid, 2.) 50 deg C, 15 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With bromine; chlorine In various solvent(s) Irradiation; | |
| With bromine; chlorine In various solvent(s) Product distribution; Irradiation; hidrogen selectivity in various photoinitiated halogenations; |

| Conditions | Yield |
|---|---|
| With sodium azide In N,N-dimethyl-formamide at 80℃; | 100% |
| With sodium azide In N,N-dimethyl-formamide at 80℃; for 24h; Inert atmosphere; | 100% |
| With sodium azide In N,N-dimethyl-formamide at 85℃; | 100% |
-
-
1666-13-3
diphenyl diselenide

-
-
103-63-9
1-phenyl-2-bromoethane

-
-
65275-36-7
(2-phenyl-ethyl) phenyl selenide

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 2h; Ambient temperature; | 100% |
| With zinc In N,N-dimethyl-formamide at 100℃; for 2h; Inert atmosphere; | 94% |
| With indium iodide In dichloromethane at 20℃; for 1h; | 89% |
| With sodium tetrahydroborate In ethanol; 1,2-dichloro-ethane at 20℃; Reduction; alkylation; | 79% |
| With triphenylphosphine; 1-pentyl-3-methylimidazolium bromide at 75℃; for 5.5h; | 75% |

| Conditions | Yield |
|---|---|
| In tert-butyl alcohol at 40℃; Rate constant; Mechanism; secondary α-deuterium isotope effects investigated; | 100% |
-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-2-bromoethane; SynPhase lantern-OC(O)-C6H4-(NC(O)C6H4NHC(S)) With 1-methyl-pyrrolidin-2-one; N-ethyl-N,N-diisopropylamine at 20℃; for 16h; Stage #2: With water; trifluoroacetic acid at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: heptanethiol With tetra-(n-butyl)ammonium iodide; caesium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 1-phenyl-2-bromoethane In N,N-dimethyl-formamide at 0 - 20℃; for 1h; | 100% |
-
-
103-63-9
1-phenyl-2-bromoethane

-
-
51721-15-4
2,2-dimethyl-propionic acid 4-(2-phthalimido-ethyl)-imidazol-1-ylmethyl ester

-
-
281197-46-4
1-pivaloyloxymethyl-3-(4-phenylethyl)-4-(2-phthalimidoethyl)imidazolium bromide

| Conditions | Yield |
|---|---|
| In acetonitrile for 72h; Heating / reflux; | 100% |

| Conditions | Yield |
|---|---|
| at 140℃; Continuous flow; neat (no solvent); | 100% |
| In acetonitrile for 18h; Reflux; Schlenk technique; | 86% |
| In toluene for 24h; Reflux; Inert atmosphere; | |
| In toluene for 24h; Reflux; Inert atmosphere; |
-
-
119072-54-7, 2769-71-3
2,6-dimethylphenyl isonitrile

-
-
28178-42-9
2,6-diisopropylphenyl isocyanate

-
-
103-63-9
1-phenyl-2-bromoethane

-
-
1392826-44-6
N-(2,6-diisopropylphenyl)-2-(2,6-dimethylphenylimino)-4-phenylbutanamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethylphenyl isonitrile; 1-phenyl-2-bromoethane With N,N,N,N,N,N-hexamethylphosphoric triamide; samarium diiodide In tetrahydrofuran at -15℃; for 3h; Stage #2: 2,6-diisopropylphenyl isocyanate In tetrahydrofuran at -78℃; | 100% |

-
-
119072-54-7, 2769-71-3
2,6-dimethylphenyl isonitrile

-
-
103-71-9
phenyl isocyanate

-
-
103-63-9
1-phenyl-2-bromoethane

-
-
1392826-54-8
2-(2,6-dimethylphenylamino)-N,4-diphenylbutanamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethylphenyl isonitrile; 1-phenyl-2-bromoethane With N,N,N,N,N,N-hexamethylphosphoric triamide; samarium diiodide In tetrahydrofuran at -15℃; for 3h; Stage #2: phenyl isocyanate In tetrahydrofuran at -78℃; for 0.5h; | 100% |

-
-
119072-54-7, 2769-71-3
2,6-dimethylphenyl isonitrile

-
-
103-71-9
phenyl isocyanate

-
-
103-63-9
1-phenyl-2-bromoethane

-
-
1392826-40-2
2-(2,6-dimethylphenylimino)-N,4-diphenylbutanamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethylphenyl isonitrile; 1-phenyl-2-bromoethane With N,N,N,N,N,N-hexamethylphosphoric triamide; samarium diiodide In tetrahydrofuran at -15℃; for 3h; Stage #2: phenyl isocyanate In tetrahydrofuran at -78℃; | 100% |

-
-
119072-54-7, 2769-71-3
2,6-dimethylphenyl isonitrile

-
-
5416-93-3
4-Methoxyphenyl isocyanate

-
-
103-63-9
1-phenyl-2-bromoethane

-
-
1392826-41-3
2-(2,6-dimethylphenylimino)-N-(4-methoxyphenyl)-4-phenylbutanamide

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethylphenyl isonitrile; 1-phenyl-2-bromoethane With N,N,N,N,N,N-hexamethylphosphoric triamide; samarium diiodide In tetrahydrofuran at -15℃; for 3h; Stage #2: 4-Methoxyphenyl isocyanate In tetrahydrofuran at -78℃; | 100% |

-
-
1124-19-2
phenyltin trichloride

-
-
103-63-9
1-phenyl-2-bromoethane

-
-
97664-42-1
tri(2-phenylethyl)(phenyl)stannane

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-2-bromoethane With iodine; magnesium In diethyl ether Inert atmosphere; Reflux; Stage #2: phenyltin trichloride In diethyl ether; n-heptane at 0 - 20℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-2-bromoethane With iodine; magnesium In tetrahydrofuran at 50℃; for 1h; Inert atmosphere; Stage #2: ortho-bromobenzaldehyde In tetrahydrofuran for 4h; Inert atmosphere; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 80℃; under 760.051 Torr; for 18h; Temperature; | 99.5% |

| Conditions | Yield |
|---|---|
| In diethylene glycol dimethyl ether; acetonitrile for 8h; Heating; | 99.1% |
-
-
103-63-9
1-phenyl-2-bromoethane

-
-
87233-69-0
1-(2-ethoxyethyl)-2-(1-piperazinyl)-1H-benzimidazole

-
-
101954-16-9
1-(2-Ethoxy-ethyl)-2-(4-phenethyl-piperazin-1-yl)-1H-benzoimidazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 65℃; for 5h; | 99% |
-
-
103-63-9
1-phenyl-2-bromoethane

-
-
603-35-0
triphenylphosphine

-
-
53213-26-6
(2-phenylethyl)triphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| at 100℃; for 2h; Addition; | 99% |
| In neat (no solvent) at 120℃; for 22h; | 99% |
| In benzene for 24h; Heating; | 92% |
-
-
103-63-9
1-phenyl-2-bromoethane

-
-
66-99-9
β-naphthaldehyde

-
-
170654-26-9
2,2,2-tris(methylthio)-1-(2-naphthyl)ethanol

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 2h; | 99% |
-
-
103-63-9
1-phenyl-2-bromoethane

| Conditions | Yield |
|---|---|
| Stage #1: pyridine-3,4-dicarboxylic acid diisopropyl ester With ammonia; sodium In tetrahydrofuran at -78℃; for 0.25h; Stage #2: 1-phenyl-2-bromoethane In tetrahydrofuran for 10h; | 99% |

| Conditions | Yield |
|---|---|
| With Iron(III) nitrate nonahydrate; 1-hydroxy-pyrrolidine-2,5-dione; oxygen In benzonitrile at 90℃; for 20h; | 99% |
| With 3,6-bis(triphenylphosphonium)cyclohexene peroxodisulfate In water; acetonitrile for 0.416667h; Heating; | 84% |
| With 1,4-bis(triphenylphosphonium)butane cerium nitrate In water; acetic acid for 0.833333h; Reflux; | 75% |
| With oxygen; Langlois reagent In acetonitrile at 25℃; under 760.051 Torr; for 12h; Irradiation; Green chemistry; | 98 %Chromat. |
-
-
103-63-9
1-phenyl-2-bromoethane

-
-
829-85-6
diphenylphosphane

-
-
5952-49-8
(2-phenylethyl)diphenylphosphane

| Conditions | Yield |
|---|---|
| With cesium hydroxide; 4 Angstroem MS In N,N-dimethyl-formamide at 23℃; for 29h; | 99% |

| Conditions | Yield |
|---|---|
| With sodium azide In ethanol at 80℃; for 8h; regioselective reaction; | 99% |
| With sodium azide In ethanol at 80℃; for 8h; | 98% |
| With sodium azide In water at 60℃; for 5h; Temperature; Green chemistry; regioselective reaction; | 97% |
-
-
1483-27-8
2,5-dimethoxythiophenol

-
-
103-63-9
1-phenyl-2-bromoethane

-
-
849919-63-7
1,4-dimethoxy-2-(2-phenethyl)thiobenzene

| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dimethoxythiophenol With potassium hydroxide In methanol for 1h; Stage #2: 1-phenyl-2-bromoethane In methanol for 0.5h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-2-bromoethane With iodine; magnesium In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: chlorodiisopropylsilane In tetrahydrofuran for 18h; Reflux; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With hydroxylamine monohydrate In methanol at 100℃; under 18100.7 Torr; for 0.166667h; Flow reactor; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: o-bromothiophenol With sodium hydride; potassium carbonate In tetrahydrofuran; N,N-dimethyl-formamide at 0℃; for 0.333333h; Stage #2: 1-phenyl-2-bromoethane In tetrahydrofuran; N,N-dimethyl-formamide at 0 - 20℃; | 99% |
(2-Bromoethyl)benzene Specification
The 2-Phenylethyl bromide with CAS registry number of 103-63-9 is also known as Benzene,(2-bromoethyl)-. The IUPAC name is 2-Bromoethylbenzene. Its EINECS registry number is 203-130-8. In addition, the formula is C8H9Br and the molecular weight is 185.08. This chemical is a colourless to yellow liquid that miscible with ether, benzene but insoluble in water. It may cause damage to health and should be stored away from oxidants. What's more, this chemical is used as pharmaceutical and pesticide intermediates, and it is also used for organic synthesis.
Physical properties about 2-Phenylethyl bromide are: (1)ACD/LogP: 3.09; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.09; (4)ACD/LogD (pH 7.4): 3.09; (5)ACD/BCF (pH 5.5): 131.12; (6)ACD/BCF (pH 7.4): 131.12; (7)ACD/KOC (pH 5.5): 1141.38; (8)ACD/KOC (pH 7.4): 1141.38; (9)#Freely Rotating Bonds: 2; (10)Index of Refraction: 1.556; (11)Molar Refractivity: 43.53 cm3; (12)Molar Volume: 135.4 cm3; (13)Surface Tension: 37.8 dyne/cm; (14)Density: 1.366 g/cm3; (15)Flash Point: 89.4 °C; (16)Enthalpy of Vaporization: 43.83 kJ/mol; (17)Boiling Point: 220.5 °C at 760 mmHg; (18)Vapour Pressure: 0.167 mmHg at 25 °C.
Preparation of 2-Phenylethyl bromide: it is prepared by reaction of phenylethanol with hydrogen bromide. Firstly, phenylethanol is heated to 110 °C and hydrogen bromide is slowly passed into for refluxing. After reaction, the reaction mixture is cooled, washed with water, 10% sodium carbonate solution and water by turns. At last, product is obtained by drying with anhydrous potassium carbonate, vacuum distillation and collecting distillate at 97-99 °C. The yield is about 90%.
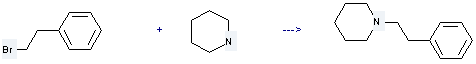
Uses of 2-Phenylethyl bromide: it is used to produce 1-phenethyl-piperidine by reaction with piperidine. The reaction occurs with solvent toluene and other condition of heating for 24 hours. The yield is about 36%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. Besides, it is harmful if swallowed. During using it, wear suitable gloves and eye/face protection. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C=C1)CCBr
2. InChI: InChI=1S/C8H9Br/c9-7-6-8-4-2-1-3-5-8/h1-5H,6-7H2
3. InChIKey: WMPPDTMATNBGJN-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 811mg/kg (811mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | National Technical Information Service. Vol. OTS0536710, |
Related Products
- (2-Bromoethyl)benzene
- 103639-04-9
- 103-64-0
- 1036401-98-5
- 1036401-99-6
- 103646-25-9
- 103646-29-3
- 103646-82-8
- 1036468-34-4
- 10364-68-8
- 10364-69-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View