-
Name
1-(2-Aminoethyl)imidazolidin-2-one
- EINECS 228-491-9
- CAS No. 6281-42-1
- Article Data20
- CAS DataBase
- Density 1.137 g/cm3
- Solubility
- Melting Point
- Formula C5H11N3O
- Boiling Point 363.1 °C at 760 mmHg
- Molecular Weight 129.162
- Flash Point 173.4 °C
- Transport Information
- Appearance
- Safety 22-26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, C
C
- Synonyms 1-(2-Aminoethyl)-2-imidazolidinone;1-(b-Aminoethyl)-2-imidazolidone;3-(2-Aminoethyl)-2-imidazolidinone;N-(2-Aminoethyl)imidazolidin-2-one;N-(2-Aminoethyl)imidazolidinone;N-(b-Aminoethyl)-N,N'-ethyleneurea;NSC 5776;[2-(2-Oxoimidazolidino)ethyl]amine;
- PSA 58.36000
- LogP -0.06270
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonia at 125 - 160℃; Concentration; Temperature; Inert atmosphere; Large scale; | 96% |
| at 210℃; | |
| at 130 - 210℃; for 3h; |

| Conditions | Yield |
|---|---|
| With cerium(IV) oxide In ethanol at 160℃; for 8h; | 36% |
| With ethanolamine at 190℃; for 0.5h; Catalytic behavior; Reagent/catalyst; |

| Conditions | Yield |
|---|---|
| With water |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 100℃; |
-
-
40778-59-4
1-(2-aminoethyl)-2-imidazolidinethione

-
-
6281-42-1
2-aminoethylimidazolidone

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; dihydrogen peroxide | |
| Multi-step reaction with 2 steps 1: chloroacetic acid 2: H2O View Scheme |
-
-
2387-20-4
1-(2-chloroethyl)imidazolidin-2-one

-
A
-
98137-74-7
2-(2-amino-4,5-dihydro-imidazol-1-yl)-ethanol

-
B
-
6281-42-1
2-aminoethylimidazolidone

| Conditions | Yield |
|---|---|
| at 100℃; |


| Conditions | Yield |
|---|---|
| With urea |
-
-
757967-03-6
bis-(2-phenylacetylamino-ethyl)-carbamic acid 4-nitro-phenyl ester

-
A
-
103-82-2
phenylacetic acid

-
B
-
6281-42-1
2-aminoethylimidazolidone

-
C
-
100-02-7
4-nitro-phenol

-
D
-
623-05-2
(4-hydroxyphenyl)methanol

-
E
-
124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| With water; penicillin-G-amidase In Chremephor EL; dimethyl sulfoxide at 37℃; pH=7.4; Kinetics; Enzymatic reaction; Aqueous phosphate buffer; |

-
-
111-40-0
1,5-diamino-3-azapentane

-
-
616-38-6
carbonic acid dimethyl ester

-
-
6281-42-1
2-aminoethylimidazolidone

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 23 - 120℃; for 4h; Inert atmosphere; | |
| With sodium methylate In methanol at 23 - 120℃; for 4h; Concentration; Solvent; Inert atmosphere; |
-
-
3699-54-5
2-oxo-imidazolidine-1-ethanol

-
-
111-40-0
1,5-diamino-3-azapentane

-
A
-
6281-42-1
2-aminoethylimidazolidone

-
B
-
111-41-1
2-(2-Aminoethylamino)ethanol

| Conditions | Yield |
|---|---|
| at 270℃; for 5h; Autoclave; Inert atmosphere; | A 30.5 %Chromat. B 16.8 %Chromat. |

-
-
120-93-4
imidazolidone

-
-
107-15-3
ethylenediamine

-
A
-
6281-42-1
2-aminoethylimidazolidone

-
B
-
623-76-7
N,N'-diethylurea

-
C
-
166532-70-3
U2TETA

-
D
-
24368-15-8
1,1'-(1,2-ethanediyl)di(2-imidazolidinone)

-
F
-
111-40-0
1,5-diamino-3-azapentane

-
G
-
112-24-3
triethylentetramine

| Conditions | Yield |
|---|---|
| With dimethylenecyclourethane at 260℃; for 1.5h; Inert atmosphere; |

-
-
120-93-4
imidazolidone

-
-
107-15-3
ethylenediamine

-
A
-
6281-42-1
2-aminoethylimidazolidone

-
B
-
166532-70-3
U2TETA

-
C
-
24368-15-8
1,1'-(1,2-ethanediyl)di(2-imidazolidinone)

-
E
-
111-40-0
1,5-diamino-3-azapentane

-
F
-
112-24-3
triethylentetramine

| Conditions | Yield |
|---|---|
| With dimethylenecyclourethane at 250℃; for 5.5h; Microwave irradiation; Inert atmosphere; |

-
-
497-25-6
dimethylenecyclourethane

-
-
120-93-4
imidazolidone

-
-
107-15-3
ethylenediamine

-
A
-
3699-54-5
2-oxo-imidazolidine-1-ethanol

-
B
-
6281-42-1
2-aminoethylimidazolidone

-
C
-
166532-70-3
U2TETA

-
D
-
24368-15-8
1,1'-(1,2-ethanediyl)di(2-imidazolidinone)

-
F
-
111-40-0
1,5-diamino-3-azapentane

-
G
-
112-24-3
triethylentetramine

| Conditions | Yield |
|---|---|
| at 250℃; for 5.5h; Inert atmosphere; |

-
-
497-25-6
dimethylenecyclourethane

-
-
120-93-4
imidazolidone

-
-
107-15-3
ethylenediamine

-
A
-
3699-54-5
2-oxo-imidazolidine-1-ethanol

-
B
-
6281-42-1
2-aminoethylimidazolidone

-
C
-
111-41-1
2-(2-Aminoethylamino)ethanol

-
D
-
111-40-0
1,5-diamino-3-azapentane

-
E
-
112-24-3
triethylentetramine

| Conditions | Yield |
|---|---|
| at 260℃; for 3h; |

-
-
120-93-4
imidazolidone

-
-
141-43-5
ethanolamine

-
-
107-15-3
ethylenediamine

-
A
-
6281-42-1
2-aminoethylimidazolidone

-
B
-
166532-70-3
U2TETA

-
C
-
24368-15-8
1,1'-(1,2-ethanediyl)di(2-imidazolidinone)

-
E
-
111-40-0
1,5-diamino-3-azapentane

-
F
-
112-24-3
triethylentetramine

| Conditions | Yield |
|---|---|
| With dimethylenecyclourethane at 250℃; for 5h; Inert atmosphere; |

-
-
6281-42-1
2-aminoethylimidazolidone

-
-
71310-21-9
11-mercaptounadecanoic acid

| Conditions | Yield |
|---|---|
| at 160℃; for 6h; Inert atmosphere; | 100% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
660870-46-2
1-(5-(methoxycarbonyl)-4-{[2-(trifluoromethyl)benzyl]oxy}thien-2-yl)-1H-benzimidazole-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In DMF (N,N-dimethyl-formamide) for 2h; | 95% |
-
-
6281-42-1
2-aminoethylimidazolidone


| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 94% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
1034658-89-3
2-chloro-4-[7-(2-chloro-pyridin-4-yl)-benzo[b]thiophen-2-yl]-pyrimidine

-
-
1034657-98-1
1-(2-{4-[7-(2-chloro-pyridin-4-yl)-benzo[b]thiophen-2-yl]pyrimidin-2-ylamino}ethyl)imidazolidin-2-one

| Conditions | Yield |
|---|---|
| In butan-1-ol at 120℃; for 5h; | 93% |

| Conditions | Yield |
|---|---|
| In water at 75 - 80℃; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20 - 100℃; for 60h; Reagent/catalyst; Solvent; Temperature; | 87.09% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
82417-45-6
dichlorobenzenesulfonyl chloride

-
-
886236-60-8
2,3-dichloro-N-[2-(2-oxoimidazolidin-1-yl)ethyl]benzenesulphonamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 87% |
-
-
77-77-0
Divinyl sulfone

-
-
6281-42-1
2-aminoethylimidazolidone

-
-
85694-70-8
1-<2-N-(1,1-dioxidothiomorpholinoethyl)>-2-imidazolidinone

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Ambient temperature; | 83% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
90826-32-7
m-isopropenyl-α,α-dimethylbenzyl carbamic acid methyl ester

-
-
137559-77-4
m-TMU

| Conditions | Yield |
|---|---|
| 1,3-diacetoxy-1,1,3,3-tetrabutyldistannoxane at 140℃; under 30 Torr; for 3h; | 83% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 80% |
-
-
6281-42-1
2-aminoethylimidazolidone

| Conditions | Yield |
|---|---|
| In acetonitrile | 78% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 60℃; for 2h; Inert atmosphere; | 75% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
893441-65-1
2-(methylsulfonyl)-4-(5-(morpholinosulfonyl)thiophen-2-yl)pyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 6h; Heating / reflux; | 72% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
1367126-25-7
C18H12FNO2

-
-
1367124-97-7
1-(2-(((6-(4-(4-fluorophenoxy)phenyl)pyridin-2-yl)methyl)amino)ethyl)imidazolidin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethylimidazolidone; C18H12FNO2 In tetrahydrofuran at 20℃; for 14h; Stage #2: With sodium tetrahydroborate In tetrahydrofuran at 20℃; for 0.333333h; | 72% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
1367126-49-5
C20H18FNO4S

-
-
1367124-99-9
1-(2-((2-(6-(4-(4-fluorophenoxy)phenyl)pyridin-2-yl)ethyl)amino)ethyl)imidazolidin-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 40℃; for 14h; | 70% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
131022-68-9
4-(5'-bromo-2'-thienyl)-2-chloropyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In isopropyl alcohol for 30h; Heating / reflux; | 68% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 5℃; for 24h; | 68% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 0.0833333h; Heating; | 67% |
-
-
6281-42-1
2-aminoethylimidazolidone

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethylimidazolidone; C19H10(2)H3F3N2O2 In methanol at 20℃; for 1h; Stage #2: With 5%-palladium/activated carbon; hydrogen In methanol at 20℃; under 760.051 Torr; for 3h; | 65% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
893434-89-4
4-(benzo[b]thiophen-2-yl)-2-(methylsulfonyl)pyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In 2-methoxy-ethanol at 100℃; | 64% |
-
-
6281-42-1
2-aminoethylimidazolidone


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 150℃; for 5h; Microwave irradiation; | 64% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
1034468-08-0
{4-[2-(2-chloro-5-fluoro-pyrimidin-4-yl)-benzo[b]thiophen-7-yl]-6-fluoro-pyridin-3-ylmethyl}-dimethylamine

-
-
1034466-20-0
1-(2-{4-[7-(5-((dimethylamino)methyl)-2-fluoro-pyridin-4-yl)benzo[b]thiophen-2-yl]-5-fluoropyrimidin-2-ylamino}ethyl)imidazolidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In butan-1-ol at 120℃; for 15h; | 63% |
-
-
6281-42-1
2-aminoethylimidazolidone


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 1h; | 61% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
41848-21-9
S-allyl isothiouronium hydrobromide

-
-
123-31-9
hydroquinone

| Conditions | Yield |
|---|---|
| With sodium iodide; sodium chloride In water; acetonitrile at 20℃; | 58% |

-
-
6281-42-1
2-aminoethylimidazolidone

-
-
151608-48-9
N-acetyl-O-[bis(benzyloxy)phosphoryl]-L-tyrosine

-
-
1268157-54-5
N-acetyl-O-[bis(benzyloxy)phosphoryl]-N'-[2-(2-oxoimidazolidin-1-yl)ethyl]-L-tyrosinamide

| Conditions | Yield |
|---|---|
| Stage #1: N-acetyl-O-[bis(benzyloxy)phosphoryl]-L-tyrosine With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 0.5h; Inert atmosphere; Stage #2: 2-aminoethylimidazolidone In dichloromethane at 0 - 20℃; for 2.16667h; Inert atmosphere; | 56% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
104-12-1
p-chlorphenylisocyanate

-
-
63874-81-7
1-[2-(2-Oxo-3-imidazolidinyl)-ethyl]-3-(4-chlorophenyl)urea

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 5℃; for 24h; | 53% |
| In tetrahydrofuran |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
1034468-28-4
4-(7-bromo-benzo[b]thiophen-2-yl)-2-chloro-5-fluoro-pyrimidine

-
-
1034468-55-7
1-{2-[4-(7-bromo-benzo[b]thiophen-2-yl)-5-fluoro-pyrimidin-2-ylamino]-ethyl}-imidazolidin-2-one

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 90℃; for 3h; | 52% |
-
-
6281-42-1
2-aminoethylimidazolidone

-
-
1034468-45-5
2-chloro-4-[7-(3-trimethylsilanylethynyl-pyridin-4-yl)-benzo[b]-thiophen-2-yl]-pyrimidine

-
-
1034468-65-9
1-(2-[4-[7-(3-trimethylsilylethynyl-pyridin-4-yl)-benzo[b]thiophen-2-yl]-pyrimidin-2-ylamino]-ethyl)-imidazolidin-2-one

| Conditions | Yield |
|---|---|
| In butan-1-ol at 120℃; for 4 - 5h; | 49% |
1-(2-Aminoethyl)-2-imidazolidone Chemical Properties
IUPAC Name: 1-(2-Aminoethyl)imidazolidin-2-one
Synonyms: 1-(2-Aminoethyl)imidazolidin-2-one ; 2-Imidazolidinone, 1-(2-aminoethyl)- ; 1-(.beta.-Aminoethyl)-2-imidazolidone
Product Categories: Imidazoles & Benzimidazoles;Imidazoles & Benzimidazoles
Molecular Structure of 1-(2-Aminoethyl)-2-imidazolidone (CAS NO.6281-42-1): 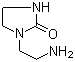
Molecular Formula of 1-(2-Aminoethyl)-2-imidazolidone (CAS NO.6281-42-1): C5H11N3O
Molecular Weight of 1-(2-Aminoethyl)-2-imidazolidone (CAS NO.6281-42-1): 129.16
CAS NO: 6281-42-1
EINECS : 228-491-9
Index of Refraction: 1.506
Surface Tension: 42.6 dyne/cm
Density: 1.137 g/cm3
Flash Point: 173.4 °C
Enthalpy of Vaporization: 60.91 kJ/mol
Boiling Point: 363.1 °C at 760 mmHg
Vapour Pressure: 1.85E-05 mmHg at 25°C
1-(2-Aminoethyl)-2-imidazolidone Production
Raw materials: Urea-->Diethylenetriamine
1-(2-Aminoethyl)-2-imidazolidone Safety Profile
Hazard Codes  Xi,
Xi, C
C
Risk Statements 34
R34:Causes burns.
Safety Statements 22-26-36/37/39-45
S22:Do not breathe dust.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
Hazard Note Irritant
Related Products
- 1-(2-Aminoethyl)-2-imidazolidone
- 628-16-0
- 628-17-1
- 6282-02-6
- 628-20-6
- 628-21-7
- 62821-89-0
- 6282-24-2
- 628-22-8
- 62823-14-7
- 62825-32-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View