-
Name
1,8-Dihydroxyanthraquinone
- EINECS 204-173-5
- CAS No. 117-10-2
- Article Data56
- CAS DataBase
- Density 1.541 g/cm3
- Solubility insoluble in water
- Melting Point 191-193 °C
- Formula C14H8O4
- Boiling Point 452.667 °C at 760 mmHg
- Molecular Weight 240.215
- Flash Point 241.691 °C
- Transport Information
- Appearance orange-brown or brown powder
- Safety 36/37-22
- Risk Codes 40
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Anthraquinone,1,8-dihydroxy- (8CI);1,8-Dihydroxy-9,10-anthracenedione;1,8-Dihydroxy-9,10-anthraquinone;1,8-Dioxyanthraquinone;Antrapurol;Chrysazin;Danthron;Danthrone;Diaquone;Laxanorm;Laxanthreen;
- PSA 74.60000
- LogP 1.87320
Synthetic route

| Conditions | Yield |
|---|---|
| With water; trifluoroacetic acid at 65℃; for 1h; | 100% |
-
-
69043-83-0
1,4-dioxo-1,2,3,4-tetrahydro-5,9,10-trihydroxyanthracene(leuco 1,4,5-trihydroxy-9,10-anthraquinone)

-
A
-
117-12-4
1,5-dihydroxyanthraquinone

-
B
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With pyrrolidine In toluene for 1.5h; Heating; | A 93% B n/a |
-
-
1432067-68-9
1,8-bis(2-phenylselenoethoxy)anthracene-9,10-dione

-
A
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
B
-
1432067-84-9
1-hydroxy-8-(2-phenylselenoethoxy)anthracene-9,10-dione

| Conditions | Yield |
|---|---|
| With iron (III) perchlorate monohydrate In dichloromethane for 2h; | A n/a B 90% |
| With copper(II) perchlorate hexahydrate In dichloromethane for 2h; | A n/a B 70% |
-
-
577975-52-1
4-hydroxy-10-imino-5-methoxymethoxy-10H-anthracen-9-one

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,4-dioxane; methanol at 20℃; for 96h; | 86% |
-
-
1182282-39-8
1,8-bis(prop-2-ynyloxy)anthracene-9,10-dione

-
A
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With iron at 160℃; for 0.0666667h; Claisen Rearrangement; Ionic liquid; | A 16% B 73% |
-
-
1393091-17-2
1-hydroxy-8-(prop-2'-ynyloxy)anthraquinone

-
A
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With iron at 160℃; for 0.2h; Claisen Rearrangement; Ionic liquid; | A 15% B 73% |
-
-
84399-87-1
8-Hydroxy-1,1,4-trimethoxy-1,4,4a,9a-tetrahydro-anthraquinone

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid | 67% |
-
-
78526-20-2
1,1-ethylenedioxy-6-hydroxy-1,4,4α,9aα-tetrahydro-9,10-anthraquinone

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With acetic acid at 100℃; for 0.333333h; | 40% |
-
-
89646-25-3
3-Hydroxy-2-(2-hydroxybenzoyl)-benzoesaeure

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid; sulfur trioxide; boric acid at 120℃; for 0.5h; | 38.4% |
| With boron trioxide; sulfuric acid at 100℃; |

-
A
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sodium acetate; pyridinium chlorochromate In dichloromethane at 20℃; for 26h; Inert atmosphere; | A n/a B 34% |

-
A
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; acetic acid In water at 20℃; for 1.5h; | A n/a B 23% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid |
-
-
1758-68-5
1,2-diamino-9,10-anthraquinone

-
A
-
117-12-4
1,5-dihydroxyanthraquinone

-
B
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium nitrite at 90 - 120℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate | |
| With quicklime; water at 180 - 200℃; | |
| With calcium hydroxide; calcium chloride at 195 - 200℃; Darstellung; im Autoklaven; |

| Conditions | Yield |
|---|---|
| With potassium acetate; acetic acid at 170℃; | |
| Multi-step reaction with 2 steps 1: water; alkali sulfite 2: quicklime; water / 180 - 200 °C View Scheme | |
| With pyridine | |
| With quicklime; water at 190 - 200℃; |

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With mixture of gaseous nitrogen oxides; sulfuric acid Erwaermen das Diazoniumsulfat mit Alkohol auf 60grad; |
-
-
52431-65-9
3-Bromojuglone

-
-
110362-30-6
1-<(trimethylsilyl)oxy>-1-methoxy-1,3-butadiene

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With hydrogenchloride 1) THF, -78 deg C, 6 h, 2) -78 deg C to r.t., 2 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With oxygen In methanol for 1.5h; Rate constant; Mechanism; different pH; half lives of decomposition; | |
| With oxygen In ethanol at 25℃; for 240h; Mechanism; other solvent; determinations of half-lives; | |
| With pyridinum sulfonate fluorochromate for 0.0222222h; Neat (no solvent); Microwave irradiation; |
-
-
1143-38-0
1,8-dihydroxy-9(10H)-anthracenone

-
A
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
B
-
31991-54-5
1,8,1',8'-tetrahydroxy-10,10'-bianthrone

| Conditions | Yield |
|---|---|
| With tetraphenylporphyrine; oxygen In methanol at -10℃; for 4h; Product distribution; Irradiation; other reagents, other solvents, other temp., no irradiation; inhibition by β-carotene; | A 67 % Chromat. B 19 % Chromat. |
| With 4-hydroxy-TEMPO benzoate; oxygen In dimethyl sulfoxide at 20℃; Kinetics; Further Variations:; Reagents; |
-
-
1143-38-0
1,8-dihydroxy-9(10H)-anthracenone

-
A
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
B
-
31991-54-5
1,8,1',8'-tetrahydroxy-10,10'-bianthrone

-
C
-
64817-79-4
1,8,10-Trihydroxy-9-anthron

| Conditions | Yield |
|---|---|
| With salcomine; oxygen In dichloromethane for 1h; Product distribution; Mechanism; in the dark; further complexes of transition metals, other solvents; |

-
-
59945-68-5
1,8,9,10-anthracenetetrol

-
-
121151-81-3
1,8-dihydroxy-9,10-anthraquinone semiquinone

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| In isopropyl alcohol Rate constant; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With rac-cysteine |
-
-
75464-11-8
1,8-dihydroxy-10-(1'-oxobutyl)-9(10H)-anthracenone

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With oxygen In methanol for 1.5h; Rate constant; Mechanism; different pH; half lives of decomposition; |
-
-
84399-87-1
(4α,4aβ,9aβ)-8-hydroxy-1,1,4-trimethoxy-1,4,4a,9a-tetrahydroanthraquinone

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid In tetrahydrofuran Ambient temperature; | 1.4 mg |
-
-
78112-67-1
1-Amino-4,5-dihydroxy-9,10-anthracenedione

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sodium hydroxide; hypophosphorous acid; sodium nitrite 1.) water, dioxan, 2.) 0 deg C, 20 min; 70 deg C, 2 h; room temperature, 10 h; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With oxygen In ethanol at 25℃; for 240h; Mechanism; other solvent; determinations of half-lives; |
-
-
151562-41-3
1,8-dihydroxy-10-(1-oxo-2-phenylethy)-9(10H)-anthracenone

-
A
-
103-82-2
phenylacetic acid

-
B
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With oxygen In ethanol at 25℃; for 240h; Mechanism; other solvent; determinations of half-lives; |
-
-
151562-48-0
1,8-Dihydroxy-10-(1-oxo-3-phenylpropyl)-9(10H)-anthracenone

-
A
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
B
-
501-52-0
3-Phenylpropionic acid

| Conditions | Yield |
|---|---|
| With oxygen In ethanol at 25℃; for 240h; Mechanism; other solvent; determinations of half-lives; |

| Conditions | Yield |
|---|---|
| In pyridine for 3h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| In pyridine for 3h; Ambient temperature; | 100% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
107-30-2
chloromethyl methyl ether

-
-
134112-14-4
1,8-bis(methoxymethoxy)-9,10-anthraquinone

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In chloroform for 16h; Heating; | 100% |
| With N-ethyl-N,N-diisopropylamine In chloroform; acetic acid methyl ester for 40h; Reflux; | 99% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
75-36-5
acetyl chloride

-
-
1963-82-2
1,8-diacetoxy-9,10-anthraquinone

| Conditions | Yield |
|---|---|
| In pyridine for 3h; Ambient temperature; | 100% |
| With pyridine | 87% |

| Conditions | Yield |
|---|---|
| In pyridine for 3h; Ambient temperature; | 100% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
108-24-7
acetic anhydride

-
-
1963-82-2
1,8-diacetoxy-9,10-anthraquinone

| Conditions | Yield |
|---|---|
| With sodium acetate Reflux; | 98% |
| at 170℃; | |
| With sodium acetate |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
6046-93-1
copper(II) acetate monohydrate

-
-
83533-69-1
bis(1,8-dihydroanthraquinonato)dicopper(II)

| Conditions | Yield |
|---|---|
| In ethanol mixture was boiled for 10 min, left stirring for 1 h at room temp.; ppt. was filtered, washed several times with ethanol, acetone, finally diethyl ether, dried in vac. over P2O5; elem. anal.; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 70℃; for 2h; pH=9; Reagent/catalyst; Temperature; | 97.8% |

-
-
64-67-5
diethyl sulfate

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
16294-26-1
1,8-diethoxyanthracene-9,10-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 2h; | 97% |
| With potassium carbonate; acetone |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
156-87-6
propan-1-ol-3-amine

-
-
858519-37-6
11-hydroxy-2-(2-hydroxyethyl)anthra[9,1-de][1,3]oxazin-7(2H)-one

| Conditions | Yield |
|---|---|
| With pyridine; copper(I) bromide at 20 - 80℃; | 97% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
80-48-8
methyl p-toluene sulfonate

-
-
6407-55-2
1,8-dimethoxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sodium carbonate at 320℃; for 0.0833333h; | 95% |
| With sodium carbonate In 1,2-dichloro-benzene Heating; | 87% |
| With sodium carbonate In various solvent(s) Yield given; |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
100-39-0
benzyl bromide

-
-
22516-60-5
1-(benzyloxy)-8-hydroxyanthracene-9,10-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide; butanone for 15h; Reflux; | 94.8% |
| With potassium carbonate In N,N-dimethyl-formamide; butanone for 20h; Inert atmosphere; Reflux; |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
108-24-7
acetic anhydride

-
-
43101-75-3
8-hydroxy-9,10-dioxo-9,10-dihydroanthracen-1-yl acetate

| Conditions | Yield |
|---|---|
| With boric acid at 90℃; for 21h; | 94% |
| With boric acid at 95℃; for 5h; | 94% |
| With boric acid at 90℃; | |
| With water; boric acid 1) 90 deg C, 10 h 2) room temp., 1 h; Yield given. Multistep reaction; | |
| With boron trioxide; (2S)-N-methyl-1-phenylpropan-2-amine hydrate; sulfuric acid 1) 3 h, 110 deg C; 2) 3 h; Yield given. Multistep reaction; |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 45h; Reflux; Inert atmosphere; | 94% |
| With 1H-imidazole In N,N-dimethyl-formamide at 25℃; for 1.25h; silylation; | 73% |

| Conditions | Yield |
|---|---|
| 93.7% | |
| With hydrogenchloride; acetic acid; tin(ll) chloride In water at 60 - 65℃; for 3h; | 92% |
| With hydrogenchloride; tin(ll) chloride In acetic acid Heating; | 90% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
77377-52-7
N-methyl-N-tert-butyldimethylsilyl-1,1,1-trifluoroacetamide

| Conditions | Yield |
|---|---|
| With tert-butyldimethylsilyl chloride In acetonitrile at 80℃; for 0.333333h; silylation; | 93% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
80-48-8
methyl p-toluene sulfonate

-
-
5539-66-2
1-hydroxy-8-methoxyanthraquinone

| Conditions | Yield |
|---|---|
| With sodium carbonate In various solvent(s) for 2h; Heating; | 93% |
| With sodium carbonate In various solvent(s) at 120℃; for 2h; | 81% |
| With sodium carbonate | 75% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
77-78-1
dimethyl sulfate

-
-
6407-55-2
1,8-dimethoxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Heating; | 92% |
| With potassium carbonate In acetone for 24.5h; Reflux; Inert atmosphere; | 90% |
| Stage #1: 1,8-dihydroxy-9,10-anthracenedione With potassium carbonate In acetone Reflux; Stage #2: dimethyl sulfate In acetone for 54h; Reflux; | 85% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
762-21-0
acetylenedicarboxylic acid diethyl ester

-
-
1018327-05-3
diethyl 2-(9,10-dihydro-1,8-dihydroxy-9,10-dioxo-anthracen-2-yl)fumarate

| Conditions | Yield |
|---|---|
| With triphenylphosphine In toluene for 24h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: copper(II) choride dihydrate; 1,8-dihydroxy-9,10-anthracenedione In methanol at 60℃; for 1h; Stage #2: With potassium hydroxide In methanol for 4h; Reflux; | 91.7% |
-
-
66086-33-7
Di-tert-butyl acetylenedicarboxylate

-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
1018327-07-5
di-tert-butyl 2-(9,10-dihydro-1,8-dihydroxy-9,10-dioxo-anthracen-2-yl)fumarate

| Conditions | Yield |
|---|---|
| With triphenylphosphine In toluene for 24h; Heating; | 91% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
106-96-7
propargyl bromide

-
-
1182282-39-8
1,8-bis(prop-2-ynyloxy)anthracene-9,10-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 24h; | 91% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; | 89% |
| With sodium carbonate In N,N-dimethyl-formamide at 20℃; | 88% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide; acetone for 120h; Inert atmosphere; Reflux; | 91% |

| Conditions | Yield |
|---|---|
| With indium; water In tetrahydrofuran; methanol at 30 - 32℃; Barbier allylation; | A 90% B 4% |


| Conditions | Yield |
|---|---|
| With indium In tetrahydrofuran; methanol; water at 29.85 - 31.85℃; | 90% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
119-26-6
(2,4-dinitro-phenyl)-hydrazine

-
-
156383-28-7
10-[(2,4-dinitrophenyl)hydrazono]-1,8-dihydroxy-10H-anthracen-9-one

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol at 50℃; for 0.5h; | 90% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
6046-93-1
copper(II) acetate monohydrate

-
-
109837-65-2, 109837-66-3, 58806-62-5
bis(1,8-dihydroanthraquinonato)copper(II)

| Conditions | Yield |
|---|---|
| In ethanol 1,8-DHAQ was dissolved in abs. ethanol, warmed to boiling, filtered, slow addn. of ethanolic soln. of Cu(CH3COO)2*H2O, mixture was stirred at room temp. for 1 h, kept in refrigerator overnight; ppt. was washed with ethanol, acetone, finally diethyl ether, dried in vac. over P2O5; elem. anal.; | 90% |
-
-
117-10-2
1,8-dihydroxy-9,10-anthracenedione

-
-
64817-82-9
1,8-dihydroxy-9,10-dihydroanthracene

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 4.25h; Reflux; | 90% |
| With aluminum (III) chloride; lithium aluminium tetrahydride In tetrahydrofuran Cooling with ice; Reflux; | 80% |
| With water; sodium hydroxide; zinc | |
| With aluminum (III) chloride; lithium aluminium tetrahydride In tetrahydrofuran Reflux; | |
| Multi-step reaction with 2 steps 1.1: acetic acid; potassium iodide; iodine / 2 h / 80 °C / Large scale 1.2: 8.5 h / 85 - 115 °C / Large scale 2.1: sodium tetrahydroborate; trifluoroacetic acid / tert-butyl methyl ether / -15 °C / Reflux View Scheme |
1,8-Dihydroxyanthraquinone Uses
The major use of 1,8-Dihydroxyanthraquinone (117-10-2) is as an intermediate in the production of alizarin and indanthrene dyestuffs.
1,8-Dihydroxyanthraquinone Consensus Reports
NTP 10th Report on Carcinogens. Reported in EPA TSCA Inventory.
1,8-Dihydroxyanthraquinone Specification
The 1,8-Dihydroxyanthraquinone, with the CAS registry number 117-10-2, is also known as 1,8-Dihydroxy-9,10-anthraquinone. It belongs to the product categories of Intermediates of Dyes and Pigments; Anthraquinones; Hydroxyanthraquinones; Aromatics; Intermediates & Fine Chemicals; Pharmaceuticals. Its EINECS registry number is 204-173-5. This chemical's molecular formula is C14H8O4 and molecular weight is 240.21. What's more, its IUPAC name is called 1,8-Dihydroxyanthracene-9,10-dione. It should be stored in a cool, dry and well-ventilated place.
Physical properties about 1,8-Dihydroxyanthraquinone are: (1)ACD/LogP: 4.003; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.93; (4)ACD/LogD (pH 7.4): 2.43; (5)ACD/BCF (pH 5.5): 551.84; (6)ACD/BCF (pH 7.4): 17.35; (7)ACD/KOC (pH 5.5): 3049.70; (8)ACD/KOC (pH 7.4): 95.86; (9)#H bond acceptors: 4; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 74.6 Å2; (13)Index of Refraction: 1.733; (14)Molar Refractivity: 62.432 cm3; (15)Molar Volume: 155.926 cm3; (16)Polarizability: 24.75×10-24cm3; (17)Surface Tension: 79.282dyne/cm; (18)Density: 1.541 g/cm3; (19)Flash Point: 241.691 °C; (20)Enthalpy of Vaporization: 73.928 kJ/mol; (21)Boiling Point: 452.667 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25 °C.
Preparation of 1,8-Dihydroxyanthraquinone: this chemical can be prepared by 3-hydroxy-2-salicyloyl-benzoic acid. This reaction needs reagent H2SO4 at temperature of 120 °C. The reaction time is 30 min. The yield is 38.4 %.
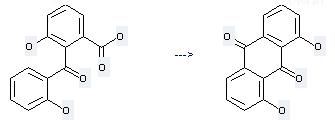
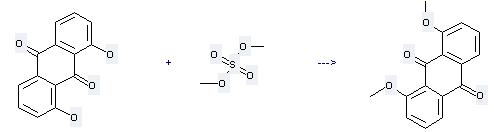
Uses of 1,8-Dihydroxyanthraquinone: (1) it is used as a stimulant laxative and used as intermediates in the production of alizarin and indanthrene dyestuffs; (2) it is used to produce other chemicals. For example, it can react with sulfuric acid dimethyl ester to get 1,8-dimethoxy-anthraquinone. The reaction occurs with reagent K2CO3 and other condition of heating for 48 hours. The yield is 77 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause damage to health and it has limited evidence of a carcinogenic effect. Therefore, you should wear suitable protective clothing and gloves.
You can still convert the following datas into molecular structure:
(1) SMILES: c1cc2c(c(c1)O)C(=O)c3c(cccc3O)C2=O
(2) InChI: InChI=1S/C14H8O4/c15-9-5-1-3-7-11(9)14(18)12-8(13(7)17)4-2-6-10(12)16/h1-6,15-16H
(3) InChIKey: QBPFLULOKWLNNW-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 500mg/kg (500mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | intravenous | > 10gm/kg (10000mg/kg) | Pharmacology and Toxicology Vol. 61, Pg. 153, 1987. | |
| mouse | LD50 | oral | > 7gm/kg (7000mg/kg) | Drug and Chemical Toxicology. Vol. 1, Pg. 89, 1977/1978. | |
| rat | LD50 | intraperitoneal | 1110mg/kg (1110mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(12), Pg. 93, 1987. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 117103-53-4
- 117106-20-4
- 117106-21-5
- 117-11-3
- 1171130-36-1
- 117119-43-4
- 1171197-20-8
- 117121-81-0
- 117-12-4
- 1171334-07-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View