-
Name
1-Phenylhexan-3-one
- EINECS 249-937-9
- CAS No. 29898-25-7
- Article Data40
- CAS DataBase
- Density 0.95 g/cm3
- Solubility
- Melting Point
- Formula C12H16O
- Boiling Point 260.8 °C at 760 mmHg
- Molecular Weight 176.258
- Flash Point 107 °C
- Transport Information
- Appearance pale yellow to colorless liquid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1-Phenyl-3-hexanone;Phenethyl propyl ketone;
- PSA 17.07000
- LogP 2.98840
Synthetic route
-
-
82297-62-9
(E)-1-phenylhex-1-en-3-one

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| Pd-C In ethyl acetate | 99.4% |
-
-
928-49-4
hex-3-yne

-
-
256379-28-9
N-(3-methyl-2-pyridyl)-N-[(E)-3-phenyl-2-propenyl]amine

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| Stage #1: hex-3-yne; N-(3-methyl-2-pyridyl)-N-[(E)-3-phenyl-2-propenyl]amine With Wilkinson's catalyst; cyclohexylamine In toluene at 130℃; for 12h; Stage #2: With water; hydrogen cation Further stages.; | 93% |

| Conditions | Yield |
|---|---|
| With 3-methylpyridin-2-ylamine; Wilkinson's catalyst; cyclohexylamine; aluminium trichloride In toluene at 130℃; for 12h; | 91% |
-
-
2180-43-0
1-phenyl-3-hexanol

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; 2-azatricyclo[3.3.1.13,7]dec-2-yloxidanyl In acetonitrile at 20℃; for 6h; chemoselective reaction; | 90% |
| With dipyridinium dichromate In dichloromethane for 8h; | 65% |
| With chromium(VI) oxide; acetic acid |

| Conditions | Yield |
|---|---|
| With 4,7-Dimethyl-1,10-phenanthroline; palladium(II) trifluoroacetate; copper (II) trifluoroacetate hydrate In 1,4-dioxane at 100℃; for 20h; Sealed tube; regioselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; bis(η3-allyl-μ-chloropalladium(II)); Tedicyp In N,N-dimethyl-formamide at 130℃; for 20h; Heck reaction; | 78% |

| Conditions | Yield |
|---|---|
| With acetylacetonatodicarbonylrhodium(l); potassium formate; potassium carbonate; triphenylphosphine In 1,2-dimethoxyethane at 130℃; for 16h; Inert atmosphere; Sealed tube; | 78% |
-
-
108-86-1
bromobenzene

-
-
18020-64-9
methyl 3-hydroxy-2-methylenehexanoate

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium formate; sodium hydrogencarbonate; Pd-benzothiazole carbene at 130℃; for 20h; | 77% |
-
-
104-53-0
3-phenyl-propionaldehyde

-
-
558-37-2
tert-butylethylene

-
-
928-49-4
hex-3-yne

-
A
-
29898-25-7
1-phenylhexan-3-one

-
B
-
5054-71-7
6,6-dimethylheptan-3-one

| Conditions | Yield |
|---|---|
| With 3-methylpyridin-2-ylamine; Wilkinson's catalyst; hexan-1-amine; 4-trifluoromethylbenzoic acid; water In toluene at 150℃; for 24h; | A 75% B 39% C 6% |


| Conditions | Yield |
|---|---|
| With trifuran-2-yl-phosphane; C48H34F6N4O4Pd2; lithium hydroxide In neat (no solvent) at 80 - 100℃; for 24h; Inert atmosphere; Schlenk technique; Sealed tube; | 72% |
| nickel In tetrahydrofuran at 76℃; for 8h; | 36% |
| With nickel(0) nanoparticles In tetrahydrofuran at 76℃; for 8h; Inert atmosphere; | 36% |

| Conditions | Yield |
|---|---|
| With tetrakis(tetrabutylammonium)decatungstate(VI); 4,4'-dimethoxyphenyl disulfide In acetonitrile at 20℃; for 18h; Sealed tube; Inert atmosphere; Irradiation; | 62% |

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; samarium diiodide In tetrahydrofuran at 0℃; for 0.5h; Barbier reaction; | 60% |
-
-
944449-56-3
[3-cyclopropyl-3-(3-iodoallyloxy)-propyl]-benzene

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| Stage #1: [3-cyclopropyl-3-(3-iodoallyloxy)-propyl]-benzene With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In benzene for 36h; Heating; Stage #2: With potassium fluoride In benzene for 24h; Further stages.; | 33% |

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 10-phenyl-9-(2,4,6-trimethylphenyl)acridinium tetrafluoroborate; diphenyldisulfane In 1,2-dichloro-ethane for 12h; Inert atmosphere; Irradiation; | 20% |
-
-
82297-62-9, 90729-78-5, 4646-80-4
1-phenylhex-1-en-3-one

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| With cyclohexene; monoaluminum phosphate; silica gel; palladium at 100℃; for 3h; | 19.6% |
| With ethanol; nickel Hydrogenation; | |
| With hydrogen Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With benzene |

| Conditions | Yield |
|---|---|
| With thorium dioxide | |
| With thorium dioxide at 460℃; |
-
-
60012-62-6
phenyl-1 hexanol-2

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| With chromic acid |
-
-
72551-30-5
(Z)-1-phenyl-3-(trimethylsiloxy)-3-hexene

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol Yield given; |
-
-
100-39-0
benzyl bromide

-
-
107-87-9
2-Pentanone

-
A
-
29898-25-7
1-phenylhexan-3-one

-
B
-
61780-85-6
3-benzyl-2-pentanone

| Conditions | Yield |
|---|---|
| With lithium chloride; manganese(ll) chloride; lithium diisopropyl amide 1.) 0 deg C, 1 h; 2.) THF, rt, 1 h then -78 deg C, 1 h; 3.) THF/NMP, -78 deg C, 1 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
113549-28-3
1-phenyl-2-(phenylsulfonyl)-hex-1-en-3-one

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| With aluminum amalgam |

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| With sodium amalgam; ethanol; acetic acid |

| Conditions | Yield |
|---|---|
| Stage #1: propyllithium With copper(l) iodide In diethyl ether at -40℃; Stage #2: hydrocinnamic acid chloride In diethyl ether at -78℃; |
-
-
944449-49-4
(3-cyclopropyl-3-prop-2-ynyloxypropyl)-benzene

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tributyltin hydride; AINB / benzene / 4 h / Heating 1.2: 0.24 g / I2 / CH2Cl2 / 12 h / 20 °C 2.1: AIBN; tributyltin hydride / benzene / 36 h / Heating 2.2: 33 percent / aq. KF / benzene / 24 h View Scheme |
-
-
52121-67-2
1-cyclopropyl-3-phenyl-1-propanol

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: cetyltrimethylammonium bromide; aq. NaOH / CH2Cl2 / 1.5 h / 20 °C 1.2: 23 percent / toluene; CH2Cl2 / 60 h / 20 °C 2.1: tributyltin hydride; AINB / benzene / 4 h / Heating 2.2: 0.24 g / I2 / CH2Cl2 / 12 h / 20 °C 3.1: AIBN; tributyltin hydride / benzene / 36 h / Heating 3.2: 33 percent / aq. KF / benzene / 24 h View Scheme |
-
-
501-52-0
3-Phenylpropionic acid

-
-
29898-25-7
1-phenylhexan-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: SOCl2; DMF / ethyl acetate 2.1: CuI / diethyl ether / -40 °C 2.2: diethyl ether / -78 °C View Scheme |
-
-
29898-25-7
1-phenylhexan-3-one

-
-
2180-43-0
1-phenyl-3-hexanol

| Conditions | Yield |
|---|---|
| With dicarbonyl-(2,4-bis(trimethylsilyl)bicyclo[3.3.0]nona-1,4-dien-3-one)[acetonitrile]iron; isopropyl alcohol at 80℃; for 18h; Inert atmosphere; | 98% |
| With diethyl ether; sodium inactive propyl-β-phenethyl-carbinol; |
-
-
75-77-4
chloro-trimethyl-silane

-
-
29898-25-7
1-phenylhexan-3-one

-
-
96-32-2
bromoacetic acid methyl ester

-
-
672284-81-0
3-trimethylsilyloxy-3-(2'-phenylethyl)caproic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: chloro-trimethyl-silane With zinc In ethyl acetate at 20 - 50℃; for 0.25h; Stage #2: 1-phenylhexan-3-one; bromoacetic acid methyl ester In ethyl acetate at 55 - 60℃; for 0.25h; Reformatsky Reaction; Stage #3: chloro-trimethyl-silane With piperazine Product distribution / selectivity; more than 3 stages; | 95% |
| Stage #1: chloro-trimethyl-silane With zinc In tetrahydrofuran at 20 - 50℃; for 0.25h; Stage #2: 1-phenylhexan-3-one; bromoacetic acid methyl ester In tetrahydrofuran at 50℃; for 0.25h; Reformatsky Reaction; Stage #3: chloro-trimethyl-silane With N,N,N,N,-tetramethylethylenediamine Product distribution / selectivity; more than 3 stages; | 92% |
| Stage #1: chloro-trimethyl-silane With zinc In tetrahydrofuran at 20 - 50℃; for 0.25h; Stage #2: 1-phenylhexan-3-one; bromoacetic acid methyl ester In tetrahydrofuran at 50℃; for 0.25h; Reformatsky Reaction; Stage #3: chloro-trimethyl-silane With piperazine Product distribution / selectivity; more than 3 stages; | 91% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 2h; Inert atmosphere; | 91% |
-
-
29898-25-7
1-phenylhexan-3-one

-
-
124-63-0
methanesulfonyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenylhexan-3-one With sodium tetrahydroborate; ethanol at 20℃; for 3h; Inert atmosphere; Stage #2: methanesulfonyl chloride With triethylamine In dichloromethane at 20℃; for 15h; Inert atmosphere; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: diazomethyl-trimethyl-silane With n-butyllithium In tetrahydrofuran; diethyl ether; hexane at -78℃; for 0.5h; Inert atmosphere; Stage #2: 1-phenylhexan-3-one In tetrahydrofuran; diethyl ether; hexane at -78 - 20℃; for 4h; Inert atmosphere; regioselective reaction; | 76% |

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide at -78℃; for 5h; | 70% |
-
-
29898-25-7
1-phenylhexan-3-one

-
-
141-97-9
ethyl acetoacetate

-
-
162174-87-0
5,6-dihydro-4-hydroxy-6-phenethyl-6-propyl-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| Multistep reaction; | 48% |
-
-
141-82-2
malonic acid

-
-
29898-25-7
1-phenylhexan-3-one

-
-
328237-98-5
2-phenethyl-2-propyl-[1,3]dioxane-4,6-dione

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic anhydride | 32% |
-
-
29898-25-7
1-phenylhexan-3-one

-
-
1077-16-3
hexylbenzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; amalgamated zinc |
-
-
29898-25-7
1-phenylhexan-3-one

-
-
16769-44-1
1-phenyl-hexan-3-one semicarbazone
-
-
29898-25-7
1-phenylhexan-3-one
-
-
29898-25-7
1-phenylhexan-3-one

-
-
105-56-6
ethyl 2-cyanoacetate

-
-
101582-27-8
2-cyano-3-phenethyl-hex-2-enoic acid ethyl ester
1-Phenylhexan-3-one Chemical Properties
Molecular Structure of 1-Phenylhexan-3-one (CAS NO.29898-25-7):
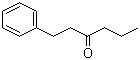
Molecular Formula: C12H16O
Molecular Weight: 176.2548
IUPAC Name: 1-Phenylhexan-3-one
Synonyms of 1-Phenylhexan-3-one (CAS NO.29898-25-7): AI3-21936 ; EINECS 249-937-9 ; 3-Hexanone, 1-phenyl-
CAS NO: 29898-25-7
Index of Refraction: 1.498
Molar Refractivity: 54.36 cm3
Molar Volume: 185.3 cm3
Surface Tension: 34.1 dyne/cm
Density: 0.95 g/cm3
Flash Point: 107 °C
Enthalpy of Vaporization: 49.85 kJ/mol
Boiling Point: 260.8 °C at 760 mmHg
Vapour Pressure of 1-Phenylhexan-3-one (CAS NO.29898-25-7): 0.012 mmHg at 25°C
Related Products
- 1-Phenylhexan-3-one
- 29898-32-6
- 2989-98-2
- 29899-95-4
- 2990-02-5
- 29901-85-7
- 2990-19-4
- 29906-54-5
- 29906-67-0
- 29908-03-0
- 29908-11-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View