-
Name
METHYL 10-UNDECENOATE
- EINECS 203-910-8
- CAS No. 111-81-9
- Article Data107
- CAS DataBase
- Density 0.88 g/cm3
- Solubility 794μg/L at 20℃
- Melting Point 26-27℃
- Formula C12H22O2
- Boiling Point 247.3 °C at 760 mmHg
- Molecular Weight 198.305
- Flash Point 99.3 °C
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols Xn,N
- Synonyms 10-Hendecenoicacid methyl ester;Maskod MNS 01-10;Methyl 10-undecenoate;Methyl10-undecylenate;Methyl undec-10-enylate;Methyl undecenoate;NSC 1273;Undecylenic acid methyl ester;
- PSA 26.30000
- LogP 3.46620
Synthetic route

| Conditions | Yield |
|---|---|
| With monoammonium 12-tungstophosphate for 12h; Heating; | 100% |
| With sulfuric acid In methanol for 6h; Reflux; | 100% |
| Stage #1: methanol; 10-undecenoic acid With thionyl chloride at 10 - 20℃; for 12h; Stage #2: With potassium carbonate In water pH=> 9; | 99% |

| Conditions | Yield |
|---|---|
| In methanol at 0 - 20℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| 98% | |
| In dichloromethane at 20℃; for 1h; Inert atmosphere; | 92% |
| With potassium carbonate | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-quinolylmethyl undec-10-enoate With ammonium formate; 1,2-bis-(diphenylphosphino)ethane; bis(dibenzylideneacetone)-palladium(0) In dimethyl sulfoxide at 50℃; for 12h; Reduction; Deprotection; Stage #2: methyl iodide With sodium carbonate In water; dimethyl sulfoxide at 20℃; for 12h; Methylation; | 95% |

| Conditions | Yield |
|---|---|
| With Amberlyst 15 at 60℃; for 48h; | 94% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide Inert atmosphere; Reflux; | 93% |
| With sodium hydroxide for 48h; Reflux; | 93% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 4h; Inert atmosphere; Reflux; | 92% |

-
-
111-81-9
Methyl 10-undecenoate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 6h; Heating; | 90% |
-
-
104367-60-4
11-tert-Butylperoxy-11-methoxy-undec-1-ene

-
-
111-81-9
Methyl 10-undecenoate

| Conditions | Yield |
|---|---|
| In methanol at 60℃; | 89% |
-
-
1227261-77-9
methyl 11-(benzo[d]thiazol-2-ylsulfonyl)-10-hydroxyundecanoate

-
-
111-81-9
Methyl 10-undecenoate

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 20℃; for 17h; | 89% |

| Conditions | Yield |
|---|---|
| With copper(II)-citrate; hexamethylenetetramine; hypophosphorous acid In water; N,N-dimethyl-formamide at 130℃; for 6h; Inert atmosphere; Green chemistry; chemoselective reaction; | 84% |

| Conditions | Yield |
|---|---|
| With Oxone at 20℃; for 4h; oxidative esterification; | 81% |

| Conditions | Yield |
|---|---|
| With iodine; potassium carbonate for 20h; Heating; | 75% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; tetrabutylammomium bromide In ethyl acetate at 20℃; for 2.85h; | 75% |
-
-
67-56-1
methanol

-
-
70995-86-7, 76249-85-9
C12H21F5O2Si(2-)*2K(1+)

-
A
-
111-81-9
Methyl 10-undecenoate

-
B
-
77149-65-6
(E)-11-Methoxy-undec-10-enoic acid methyl ester

| Conditions | Yield |
|---|---|
| With oxygen; copper diacetate for 21h; Ambient temperature; | A 16% B 67% |


| Conditions | Yield |
|---|---|
| With pyridine; copper (II) carbonate hydroxide at 90℃; for 24h; Chan-Lam Coupling; | 60% |
-
-
3903-40-0
12-methoxy-12-oxododecanoic acid

-
A
-
1731-86-8
methyl undecanoate

-
B
-
111-81-9
Methyl 10-undecenoate

-
C
-
22399-98-0
docosanedioic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With potassium methanolate In methanol at 40 - 45℃; electrolysis: anode: platinum foil; current source: galvanostat; current density 220 mA cm-2; current consumption: 1.25 F mol-1; cell voltage 40-70 V; Yield given; | A n/a B n/a C 53% |
| With potassium methanolate In methanol at 40 - 45℃; electrolysis: anode: platinum foil; current source: galvanostat; current density 220 mA cm-2; current consumption: 1.25 F mol-1; cell voltage 40-70 V; Yields of byproduct given; | A n/a B n/a C 53% |


| Conditions | Yield |
|---|---|
| With sand for 0.75h; | A n/a B 49% |

| Conditions | Yield |
|---|---|
| With Pt/HCl/F-ZSM-5 In water at 350 - 480℃; Reagent/catalyst; Temperature; | A 26.4% B 48.3% |
| at 120 - 170℃; under 145 Torr; Die Spaltung verlaeuft fast quantitativ, wenn ueber mit Borax getraenktem Bimstein destilliert wird; | |
| at 550℃; |
-
-
34574-67-9
methyl 11-bromo-10-hydroxyundecanoate

-
A
-
111-81-9
Methyl 10-undecenoate

-
B
-
54704-39-1
(+/-)-10-Hydroxyundecansaeure-methylester

-
C
-
18993-09-4
methyl 10-oxoundecanoate

-
D
-
64749-27-5
methyl 8-undecenoate

| Conditions | Yield |
|---|---|
| With chlorotris(triphenylphosphine)cobalt(I) for 6h; Ambient temperature; Yield given. Further byproducts given; | A n/a B 42% C 44% D n/a |

-
-
34574-67-9
methyl 11-bromo-10-hydroxyundecanoate

-
A
-
111-81-9
Methyl 10-undecenoate

-
B
-
54704-39-1
(+/-)-10-Hydroxyundecansaeure-methylester

-
C
-
18993-09-4
methyl 10-oxoundecanoate

-
D
-
100288-72-0
methyl 8-undecenote

| Conditions | Yield |
|---|---|
| With chlorotris(triphenylphosphine)cobalt(I) In benzene at 60℃; for 7.5h; Yield given; | A n/a B 42% C 44% D n/a |

-
-
27367-70-0
(E)-2-hydroxyiminocyclododecanone

-
A
-
111-81-9
Methyl 10-undecenoate

-
B
-
27335-49-5
(Z)-2-hydroxyiminocyclododecanone

-
C
-
27336-68-1, 80845-16-5
12-hydroxybicyclo<8.2.0>dodecan-11-one oxime

-
D
-
76185-10-9
(2-oxocyclodecane)acetonitrile

| Conditions | Yield |
|---|---|
| In methanol at 28℃; for 4h; Irradiation; Yields of byproduct given; | A n/a B 18% C n/a D n/a |
| In methanol at 28℃; for 12h; Irradiation; | A 13% B n/a C n/a D n/a |
| In methanol at 28℃; for 12h; Irradiation; | A n/a B n/a C n/a D 5% |
| In methanol at 28℃; for 6h; Irradiation; Yield given; |

-
-
27367-70-0
(E)-2-hydroxyiminocyclododecanone

-
A
-
111-81-9
Methyl 10-undecenoate

-
B
-
76185-10-9
(2-oxocyclodecane)acetonitrile

| Conditions | Yield |
|---|---|
| In methanol for 12h; Irradiation; | A 13% B n/a |
| In methanol for 12h; Irradiation; | A n/a B 5% |

| Conditions | Yield |
|---|---|
| Destillation unter normalem Druck; ricinolic acid methyl ester; |

-
-
111-81-9
Methyl 10-undecenoate

| Conditions | Yield |
|---|---|
| durch thermische Zersetzung; |
-
-
41989-07-5
methyl ricinoleate

-
-
111-81-9
Methyl 10-undecenoate

| Conditions | Yield |
|---|---|
| (methylation); | |
| Multi-step reaction with 2 steps 1: PCl5 / benzene / Heating 2: 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1: thionyl chloride / 3 h / Heating 2: 2 h / Reflux View Scheme |
-
-
112507-35-4
12-Methylricinolsaeure-methylester

-
-
111-81-9
Methyl 10-undecenoate

| Conditions | Yield |
|---|---|
| (pyrolysis); |
-
-
6287-90-7
11-bromo-undecanoic acid methyl ester

-
A
-
111-81-9
Methyl 10-undecenoate

-
B
-
37973-85-6
methyl (E) 9-undecenoate

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; n-butyllithium; 1,8-diazabicyclo[5.4.0]undec-7-ene; triphenylphosphine In tetrahydrofuran; hexane Product distribution; Mechanism; Ambient temperature; other halides; var. reagents; |
-
-
111-81-9
Methyl 10-undecenoate

-
-
14811-73-5
10-oxo-decanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With 3,3'-dimethoxy-(1,1'-biphenyl)-4,4'-diamine; oxygen In acetonitrile for 12h; Ambient temperature; Irradiation; | 100% |
| With ozone; triphenylphosphine In methanol; dichloromethane at -78 - 20℃; for 6h; | 100% |
| With sodium periodate; osmium(VIII) oxide; tetra(n-butyl)ammonium hydrogensulfate In water for 22h; Ambient temperature; | 97% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
22663-09-8
methyl 9-(oxiran-2-yl)nonanoate

| Conditions | Yield |
|---|---|
| With Oxone; ethylene diamine tetraacetic acid tetrasodium salt; sodium hydrogencarbonate In acetonitrile at 0℃; for 1h; | 100% |
| With dodecatungstophosphoric acid; dihydrogen peroxide; Aliquat 336 In chloroform at 65℃; for 5h; | 99% |
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0 - 20℃; for 12h; | 93% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
18993-09-4
methyl 10-oxoundecanoate

| Conditions | Yield |
|---|---|
| With oxygen; copper(l) chloride; palladium dichloride In tetrahydrofuran; water at 20℃; for 10h; | 100% |
| With water; oxygen; copper(l) chloride In tetrahydrofuran at 20℃; under 759.826 Torr; for 36h; Wacker-Tsuji Olefin Oxidation; | 96% |
| With tetraethylammonium chloride; oxygen; copper dichloride; palladium dichloride In N,N-dimethyl-formamide at 20℃; for 6h; Product distribution; other solvents, reagents, reagents ratios, temperature, times; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: Methyl 10-undecenoate With water; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In water | 100% |
| Acidic conditions; | 44.8% |
| Hydrolysis; |
-
-
111-81-9
Methyl 10-undecenoate

-
-
624-48-6
dimethyl cis-but-2-ene-1,4-dioate

-
A
-
70086-90-7
dimethyl dodec-2-en-1,12-dioate

-
B
-
292638-85-8
acrylic acid methyl ester

| Conditions | Yield |
|---|---|
| With [1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro-(3-phenyl-1H-inden-1-ylidene)(tricyclohexylphosphine)ruthenium(II) In toluene at 50℃; for 3h; Reagent/catalyst; Temperature; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In tetrahydrofuran; hexane at -78℃; for 5h; | 99% |
| With C17H16BrMnNO3P; potassium tert-butylate; hydrogen In 1,4-dioxane at 100℃; under 37503.8 Torr; for 6h; Autoclave; chemoselective reaction; | 98% |
| With isopropyl alcohol In hexane at 0 - 20℃; for 0.25h; Bouveault-Blanc Reduction; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With magnesium nitride In methanol at 80℃; for 24h; | 99% |
| With ammonia In methanol for 120h; | 48% |

| Conditions | Yield |
|---|---|
| With C40H56N2RuSi4; hydrogen In toluene at 25℃; under 760.051 Torr; for 1.5h; Schlenk technique; | 99% |
| With C40H56FeN2Si4(2-); hydrogen In 1,2-dimethoxyethane at 80℃; under 7600.51 Torr; for 2h; Schlenk technique; Autoclave; | 99% |
| With hydrogen; palladium dichloride In dichloromethane at 20℃; under 760 Torr; | 98% |
-
-
887144-94-7
Togni's reagent II

-
-
111-81-9
Methyl 10-undecenoate

-
-
1344711-01-8
(E)-methyl 12,12,12-trifluorododec-9-enoate

| Conditions | Yield |
|---|---|
| With copper(l) chloride In methanol at 70℃; for 0.166667h; Sealed tube; Inert atmosphere; | 99% |
| With tetrakis(actonitrile)copper(I) hexafluorophosphate In methanol at 0 - 20℃; for 23.25h; Inert atmosphere; optical yield given as %de; | 79% |

| Conditions | Yield |
|---|---|
| With C20H22N2ORh(1+)*C24H20B(1-); hydrogen In toluene at 85℃; under 37503.8 Torr; for 6h; chemoselective reaction; | 99% |

-
-
111-81-9
Methyl 10-undecenoate

-
-
53481-02-0, 63318-27-4, 63318-28-5
dimethyl 10-eicosenedioate

| Conditions | Yield |
|---|---|
| With Grubbs catalyst first generation at 20 - 50℃; for 3h; | 98% |
| With C29H36N3O4Ru In tetrahydrofuran at 35℃; for 12h; Inert atmosphere; | 94% |
| With silica immobilized second generation Hoveyda-Grubbs catalyst In hexane at 59.84℃; for 5h; | 85% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
75-16-1
methylmagnesium bromide

-
-
34386-60-2
11-hydroxy-11-methyl-dodec-1-ene

| Conditions | Yield |
|---|---|
| Stage #1: Methyl 10-undecenoate; methylmagnesium bromide In tetrahydrofuran at -78 - 20℃; for 20h; Inert atmosphere; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 0℃; | 98% |
| In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With eosin; caesium carbonate In N,N-dimethyl acetamide at 20℃; for 48h; Inert atmosphere; Sealed tube; Irradiation; | 98% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
37973-85-6
methyl (E) 9-undecenoate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 10h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With dilauryl peroxide In neat (no solvent) at 85℃; Sealed tube; | 97% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
109-55-7
1-amino-3-(dimethylamino)propane

-
-
66654-01-1
N-[3-(dimethylamino)propyl]undec-10-enamide

| Conditions | Yield |
|---|---|
| With sodium methylate at 150℃; for 4h; Reagent/catalyst; Temperature; | 96.3% |
-
-
998-30-1
Triethoxysilane

-
-
111-81-9
Methyl 10-undecenoate

-
-
18505-40-3
11-triethoxysilanylundecanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex at 60℃; for 6h; | 96% |
| dihydrogen hexachloroplatinate for 46h; Heating; |
-
-
111-81-9
Methyl 10-undecenoate

-
-
109872-87-9
methyl difluoroiodoacetate

-
-
133950-95-5
2,2-Difluoro-4-iodo-tridecanedioic acid dimethyl ester

| Conditions | Yield |
|---|---|
| copper In N,N-dimethyl-formamide for 6h; Ambient temperature; | 96% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
456-56-4
phenyl trifluoromethylsulfide

-
-
141023-04-3
1,1,1-trifluoro-11-dodecen-2-one

| Conditions | Yield |
|---|---|
| With Triethylgermyl-natrium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide at -60℃; for 1.5h; | 96% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
164353-34-8
[1',1'-2H2]-undec-10-en-1-ol

| Conditions | Yield |
|---|---|
| With lithium aluminium deuteride In tetrahydrofuran at 0 - 25℃; | 96% |
| With ethyl [2]alcohol; sodium In hexane; mineral oil at 0℃; for 0.0833333h; Inert atmosphere; | 95% |
| With ethyl [2]alcohol; sodium In hexane; mineral oil at 0 - 20℃; for 0.166667h; Bouveault-Blanc Reduction; Inert atmosphere; | 95% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
892846-46-7
methyl 11-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)undecanoate

| Conditions | Yield |
|---|---|
| With trihexyltetradecylP(+) dodecylbenzene sulfonate; N-pentyl-4-methylpy 7,8-(Cy2P)2-7,8-nido-C2B9H10; Ir nanoparticles In dichloromethane (Ar); react. alkene with boron compd. in the presence of Ir nanoparticles and boronate in CH2Cl2 and trihexyltetradecylphosphonium dodecylbenzene sulfonate at room temp. for 12 h with stirring; evapn., extn. with Et2O, concn. in vac., chromy. (SiO2, hexane/Et2O 8:1 vol.); elem. anal.; | 96% |
| With 1-hexyl-3-methylimidazolium triflate; N-pentyl-4-methylpy 7,8-(Cy2P)2-7,8-nido-C2B9H10; Ir nanoparticles In dichloromethane (Ar); react. alkene with boron compd. in the presence of Ir nanoparticles and boronate in CH2Cl2 and 1-hexyl-3-methylimidazolium trifluoromethanesulfonate at room temp. for 12 h with stirring; evapn., extn. with Et2O, concn. in vac., chromy. (SiO2, hexane/Et2O 8:1 vol.); | 93% |
| With trihexyltetradecylP(+) dodecylbenzene sulfonate; catalyst: Ir/1,2-bis(diphenylphosphino)ethane In dichloromethane (Ar); react. alkene with boron compd. in the presence of Ir nanoparticles and diphosphine in CH2Cl2 and trihexyltetradecylphosphonium dodecylbenzene sulfonate at room temp. for 12 h with stirring; evapn., extn. with Et2O, concn. in vac., chromy. (SiO2, hexane/Et2O 8:1 vol.); | 88% |
| With MesBIPCoCl2; sodium t-butanolate at 25℃; for 1h; | 70% |
| With [(1,3-bis(2,4,6-trimethylphenyl) imidazol-2-ylidene)Fe(CO)4] In neat (no solvent) at 20℃; for 24h; Inert atmosphere; UV-irradiation; Schlenk technique; regioselective reaction; | 52% |
-
-
2314-97-8
iodotrifluoromethane

-
-
111-81-9
Methyl 10-undecenoate

-
-
125081-42-7
methyl 12,12,12-trifluoro-10-iodododecanoate

| Conditions | Yield |
|---|---|
| With Raney Ni (W-2) In methanol at 80℃; for 20h; Addition; | 95% |
| With triethyl borane In hexane at -24℃; for 3.5h; | 86% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
60-24-2
2-hydroxyethanethiol

-
-
60631-70-1
methyl 11-(2-hydroxyethylthio)undecanoate

| Conditions | Yield |
|---|---|
| With 2,2-dimethoxy-2-phenylacetophenone In acetonitrile at 20℃; Visible light; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: methanol; Methyl 10-undecenoate With ozone In methanol at 0℃; Stage #2: With semicarbazide hydrochloride at 0 - 20℃; Reagent/catalyst; Inert atmosphere; | 95% |
-
-
111-81-9
Methyl 10-undecenoate

-
-
1576-98-3, 18621-75-5, 25260-60-0
1,4-diacetoxybut-2-ene

| Conditions | Yield |
|---|---|
| Stage #1: Methyl 10-undecenoate; 1,4-diacetoxybut-2-ene With [1,3-bis(2,6-di-isopropylphenyl)-2-imidazolidinylidene]dichloro(o-isopropoxyphenylmethylene)ruthenium(II) In dichloromethane at 45℃; for 6h; Inert atmosphere; Glovebox; Schlenk technique; Stage #2: With methanol; potassium hydroxide In dichloromethane at 20℃; | 94% |
10-Undecenoic acid,methyl ester Specification
The 10-Undecenoic acid,methyl ester is an organic compound with the formula C12H22O2. The IUPAC name of this chemical is methyl undec-10-enoate. With the CAS registry number 111-81-9, it is also named as Methyl 10-undecenate. The product's category is Aromatic Esters. Besides, it should be stored in a cool and well-ventilated place.
Physical properties about 10-Undecenoic acid,methyl ester are: (1)ACD/LogP: 4.45; (2)ACD/LogD (pH 5.5): 4.45; (3)ACD/LogD (pH 7.4): 4.45; (4)ACD/BCF (pH 5.5): 1416.9; (5)ACD/BCF (pH 7.4): 1416.9; (6)ACD/KOC (pH 5.5): 6270.86; (7)ACD/KOC (pH 7.4): 6270.86; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 10; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.438; (12)Molar Refractivity: 59.14 cm3; (13)Molar Volume: 225.1 cm3; (14)Polarizability: 23.44×10-24cm3; (15)Surface Tension: 29.1 dyne/cm; (16)Density: 0.88 g/cm3; (17)Flash Point: 99.3 °C; (18)Enthalpy of Vaporization: 48.45 kJ/mol; (19)Boiling Point: 247.3 °C at 760 mmHg; (20)Vapour Pressure: 0.0259 mmHg at 25°C.
Preparation of 10-Undecenoic acid,methyl ester: this chemical can be prepared by methanol and undec-10-enoic acid. This reaction is a kind of Esterification. This reaction will need reagent H2SO4. The reaction time is 3 hours by heating.
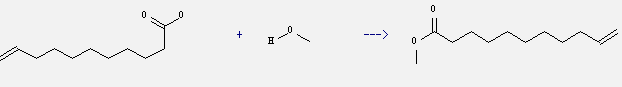
Uses of 10-Undecenoic acid,methyl ester: it can be used to produce 10-oxo-decanoic acid methyl ester. It will need reagent ozone.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC)CCCCCCCC\C=C
(2)InChI: InChI=1/C12H22O2/c1-3-4-5-6-7-8-9-10-11-12(13)14-2/h3H,1,4-11H2,2H3
(3)InChIKey: KISVAASFGZJBCY-UHFFFAOYAA
(4)Std. InChI: InChI=1S/C12H22O2/c1-3-4-5-6-7-8-9-10-11-12(13)14-2/h3H,1,4-11H2,2H3
(5)Std. InChIKey: KISVAASFGZJBCY-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View