-
Name
1H-Indazol-5-ol
- EINECS
- CAS No. 15579-15-4
- Article Data10
- CAS DataBase
- Density 1.434 g/cm3
- Solubility
- Melting Point 186-191°C
- Formula C7H6N2O
- Boiling Point 366.5 °C at 760 mmHg
- Molecular Weight 134.137
- Flash Point 175.5 °C
- Transport Information
- Appearance
- Safety 26-39
- Risk Codes 22-41
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 5-Indazolol(5CI);5-Hydroxy-1H-indazole;5-Hydroxyindazole;NSC 520104;
- PSA 48.91000
- LogP 1.26850
Synthetic route
-
-
94444-96-9
5-methoxy-1H-indazole

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 20℃; for 10h; | 71% |
-
-
19335-11-6
1H-indazol-5-ylamine

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With sulfuric acid at 170 - 180℃; | |
| With sulfuric acid at 200 - 205℃; unter Druck; | |
| Stage #1: 1H-indazol-5-ylamine With sulfuric acid In water at 180℃; for 15h; Stage #2: With sodium hydroxide In water pH=> 10; |
-
-
36174-07-9
N,O-diacetyl-5-hydroxy-1H-indazole

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 80℃; for 5h; | 7.99 g |
-
-
2835-99-6
4-amino-3-methylphenol

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium acetate; isoamyl nitrite / CHCl3 / 18 h / 80 °C 2: 7.99 g / HCl / methanol / 5 h / 80 °C View Scheme |
-
-
5401-94-5
5-nitroindazole

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Raney nickel / Hydrogenation 2: aqueous H2SO4 / 170 - 180 °C View Scheme |
-
-
543-87-3
isopentyl nitrate

-
-
2835-99-6
4-amino-3-methylphenol

-
-
144-55-8
sodium hydrogencarbonate

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With potassium acetate; acetic anhydride In hydrochloric acid-methanol; water; chlorobenzene |

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid; sodium nitrite / water / 20 °C 2: boron tribromide / dichloromethane / 10 h / 20 °C View Scheme |
-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
1254473-74-9
5-(tert-butyl-dimethyl-silanyloxy)-1H-indazole

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 18℃; for 3.5h; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 18℃; for 3.5h; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide at 25℃; for 18h; | 99% |
-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
105-36-2
ethyl bromoacetate

-
-
30226-16-5
1H-indazol-5-yloxyacetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5-hydroxy-1H-indazole; ethyl bromoacetate With potassium carbonate In acetone for 16h; Inert atmosphere; Reflux; Stage #2: With sodium hydroxide In methanol at 20℃; for 3h; Inert atmosphere; | 99% |
-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrahydrofuran at 20℃; for 14h; | 91% |
| With N-Bromosuccinimide In tetrahydrofuran at 25℃; for 12h; | 90% |
-
-
1351167-84-4
(R)-tert-butyl 4-(3-(methylsulfonyloxy)-2-oxopyrrolidin-1-yl)piperidin-1-carboxylate

-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
1351168-51-8
(S)-tert-butyl 4-(3-(1H-indazol-5-yloxy)-2-oxopyrrolidin-1-yl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 130℃; | 90% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrahydrofuran at 0℃; for 2.5h; | 89% |
-
-
3934-20-1
2,6-Dichloropyrimidine

-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
1595291-23-8
5-((2-chloropyrimidin-4-yl)oxy)-1H-indazole

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 80℃; | 87% |
| With triethylamine In ethanol at 80℃; | 87% |
| With triethylamine In ethanol at 80℃; | |
| With triethylamine In ethanol at 80℃; |
-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
478834-25-2
4-chloro-1H-indazol-5-ol

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In tetrahydrofuran at 20 - 50℃; for 8h; | 86% |

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With copper(l) chloride In tetrahydrofuran at 50℃; for 12h; Inert atmosphere; Sealed tube; regioselective reaction; | 82% |
-
-
6780-49-0
Ethyl N-phenylformimidate

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| at 175℃; for 2h; | 76% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-hydroxy-1H-indazole; C19H22F3IO2 With copper(l) chloride In tetrahydrofuran at 80℃; for 12h; Inert atmosphere; Stage #2: acetyl chloride With triethylamine In tetrahydrofuran at 20℃; for 4h; Stage #3: With N-chloro-succinimide In tetrahydrofuran at 20℃; for 4h; | 75.2% |

-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
618-51-9
3-Iodobenzoic acid

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; tert-butyl XPhos In 2-methyltetrahydrofuran at 70℃; for 1h; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: C16H16F3IO4S; 5-hydroxy-1H-indazole With copper(l) chloride In tetrahydrofuran at 80℃; for 12h; Inert atmosphere; Sealed tube; Stage #2: acetyl chloride With triethylamine at 20℃; for 4h; Inert atmosphere; Sealed tube; Stage #3: With N-chloro-succinimide at 20℃; for 4h; Inert atmosphere; Sealed tube; | 75% |


-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
1430211-17-8
5-(2-((5-(2-(3,4-dimethoxyphenyl)propan-2-yl)-1-(4-fluorophenyl)-1H-imidazol-2-yl)thio)ethoxy)-1H-indazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide; acetonitrile at 70℃; for 5h; Temperature; Inert atmosphere; | 74% |

| Conditions | Yield |
|---|---|
| Porcine Brain NADase(EC 3.2.2.5) In water; dimethyl sulfoxide for 15h; Tris-HCl; | 73% |
-
-
352-33-0
1-Chloro-4-fluorobenzene

-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
1034156-20-1
1-(4-fluorophenyl)-1H-indazol-5-ol

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; tert-butyl XPhos In 2-methyltetrahydrofuran at 73℃; for 15h; Industrial scale; | 72% |

| Conditions | Yield |
|---|---|
| In toluene at 100℃; | 70% |


| Conditions | Yield |
|---|---|
| In ethanol at 120℃; for 0.75h; Microwave irradiation; | 67% |


| Conditions | Yield |
|---|---|
| In ethanol at 120℃; for 0.75h; Microwave irradiation; | 65% |

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 12h; Inert atmosphere; | 63% |

| Conditions | Yield |
|---|---|
| In toluene at 100℃; | 62% |


| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 72h; | 59.3% |
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 6h; | |
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 26h; |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In tetrahydrofuran; dichloromethane at 20℃; for 72h; Inert atmosphere; | 54% |
| With methanesulfonic acid In tetrahydrofuran; dichloromethane at 20℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 18h; | 54% |
-
-
5402-55-1
2-thiophenethanol

-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
1338795-77-9
5-(2-(thiophen-2-yl)ethoxy)-1H-indazole

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran; toluene at 0℃; for 4h; | 52% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran; toluene at 0℃; for 4h; Inert atmosphere; | 52% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; for 5h; | 52% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; |
-
-
936850-02-1
6-chloro-N-(5-methyl-1H-pyrazol-3-yl)-2-(methylsulfonyl)pyrimidin-4-amine

-
-
15579-15-4
5-hydroxy-1H-indazole

-
-
76-05-1
trifluoroacetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 6-chloro-N-(5-methyl-1H-pyrazol-3-yl)-2-(methylsulfonyl)pyrimidin-4-amine; 5-hydroxy-1H-indazole With potassium tert-butylate In tert-butyl alcohol at 50℃; Stage #2: trifluoroacetic acid | 52% |

-
-
2081-44-9
Tetrahydro-pyran-4-ol

-
-
15579-15-4
5-hydroxy-1H-indazole

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran; toluene at 0 - 20℃; | 47% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; | 46% |
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; | 46% |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 40℃; for 2h; | 38% |
1H-Indazol-5-ol Chemical Properties
IUPAC Name: 1H-indazol-5-ol
Synonyms of 1H-Indazol-5-ol (CAS NO.15579-15-4): 5-Hydroxy indazole ; Hydroxy-5 indazole
Product Categories: Alcohols and Derivatives;Heterocycles;pharmacetical
CAS NO: 15579-15-4
Molecular Formula: C7H6N2O
Molecular Weight : 134.135340 g/mol
Molecular Structure: 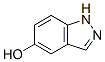
H bond acceptors: 3
H bond donors: 2
Freely Rotating Bonds: 1
Polar Surface Area: 27.05Å2
Index of Refraction: 1.76
Molar Refractivity: 38.5 cm3
Molar Volume: 93.5 cm3
Surface Tension: 80.1 dyne/cm
Density: 1.434 g/cm3
Flash Point: 175.5 °C
Enthalpy of Vaporization: 63.7 kJ/mol
Boiling Point: 366.5 °C at 760 mmHg
Vapour Pressure: 6.9E-06 mmHg at 25°C
1H-Indazol-5-ol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 1gm/kg (1000mg/kg) | French Medicament Patent Document. Vol. #7631M, |
1H-Indazol-5-ol Safety Profile
Hazard Cods:  Xi
Xi
Hazard Note: Irritant
Related Products
- 1H-Indazol-5-ol
- 15580-32-2
- 155814-20-3
- 155814-22-5
- 155815-92-2
- 155815-95-5
- 1558-17-4
- 155819-07-1
- 155824-29-6
- 1558-24-3
- 1558-25-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View