-
Name
2-PHENYLPYRROLE
- EINECS 1592732-453-0
- CAS No. 3042-22-6
- Article Data145
- CAS DataBase
- Density 1.076 g/cm3
- Solubility
- Melting Point 130℃
- Formula C10H9N
- Boiling Point 302.4 °C at 760 mmHg
- Molecular Weight 143.188
- Flash Point 124.6 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Pyrrole,2-phenyl- (6CI,7CI,8CI);2-Phenyl-1H-pyrrole;2-Phenylpyrrole;NSC 94963;2-phenyl-1H-pyrrole;
- PSA 15.79000
- LogP 2.68170
Synthetic route
-
-
199276-69-2
(4-azidobut-1-yn-1-yl)benzene

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; phenylmercuric acetate In nitromethane at 20℃; for 0.0833333h; Inert atmosphere; | 99% |
| With 2,6-di-tert-butyl-4-methylpyridine; platinum(IV) chloride In ethanol for 6h; Heating; | 74% |
| With silver hexafluoroantimonate; (bis(diphenylphosphino)methane)bis(chlorogold(I)) In dichloromethane at 35℃; Schmidt reaction; | 68% |

| Conditions | Yield |
|---|---|
| With acetic acid In methanol at 20℃; for 2h; Inert atmosphere; Molecular sieve; | 99% |

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With zinc In ethanol for 16h; Inert atmosphere; Reflux; | 99% |
-
-
132868-20-3
6-Phenoxy-3-phenyl-5,6-dihydro-4H-[1,2]oxazine

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With triiron dodecarbonyl In 1,2-dichloro-ethane at 80℃; for 20h; | 98% |
-
-
85868-20-8
O-Vinylacetophenonoxime

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With potassium hydroxide; dimethyl sulfoxide at 95℃; for 1.33333h; | 96% |

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With sodium ethanolate In dimethyl sulfoxide at 100℃; for 24h; | 94% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; sodium pentafluorophenolate In various solvent(s) at 130℃; for 12h; | 93% |

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With tris-(4,4’-di-tert-butyl-2,2’-bipyridine)ruthenium(II) hexafluorophosphate In chloroform at 20℃; for 3h; Concentration; Schlenk technique; Inert atmosphere; Irradiation; | 93% |
-
-
132868-21-4
6-butoxy-3-phenyl-1,2-oxazine

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With triiron dodecarbonyl In 1,2-dichloro-ethane at 80℃; for 20h; | 91% |
-
-
109-97-7
pyrrole

-
-
591-50-4
iodobenzene

-
A
-
3042-22-6
2-phenyl-1H-pyrrole

-
B
-
27649-43-0
3-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: pyrrole With sodium hydride In tetrahydrofuran Stage #2: iodobenzene With zinc(II) chloride; johnphos; palladium diacetate In tetrahydrofuran at 100℃; for 21h; | A 91% B n/a |
| With potassium tert-butylate In dimethyl sulfoxide at 80℃; for 22h; Sealed tube; Inert atmosphere; regioselective reaction; |


| Conditions | Yield |
|---|---|
| With [10%-Pd/Al2O3] In 5,5-dimethyl-1,3-cyclohexadiene at 150℃; for 1.5h; Kinetics; Reagent/catalyst; Time; Concentration; Inert atmosphere; | A 90% B n/a |
| With [10%-Pd/Al2O3] In 5,5-dimethyl-1,3-cyclohexadiene at 150℃; for 2h; | A 79% B n/a |
-
-
161564-59-6
acetophenone O-vinyloxime

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tert-butyl alcohol for 5h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; magnesium oxide; triphenylphosphine In 1,4-dioxane at 150℃; | 86% |
| With [Rh(coe)2Cl]2; tris(para-trifluoromethyl)phenyl phosphine In 1,4-dioxane at 120℃; | 78% |
| With C52H44CoN4O4; potassium hydroxide; tert-butyl alcohol at 200℃; for 0.5h; Inert atmosphere; Darkness; regioselective reaction; | 69% |
-
-
591-50-4
iodobenzene

-
-
135884-31-0
N-boc-2-pyrroleboronic acid

-
A
-
3042-22-6
2-phenyl-1H-pyrrole

-
B
-
163525-97-1
tert-butyl 2-phenyl-1H-pyrrole-1-carboxylate

| Conditions | Yield |
|---|---|
| With methanol; tetrakis(triphenylphosphine) palladium(0); caesium carbonate In toluene for 24h; Suzuki coupling; Reflux; Inert atmosphere; | A 7% B 86% |

-
-
1616603-16-7
1-phenylbut-3-en-1-one acetyloxime

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With copper(I) bromide In toluene at 120℃; Solvent; Reagent/catalyst; Inert atmosphere; | 86% |
-
-
120256-08-8
Dimethyl-(2-phenyl-pyrrol-1-yl)-amine

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In methanol; water under 3102.9 Torr; for 48h; Ambient temperature; | 85% |
-
-
58042-93-6
2-phenyl-1-vinyl-1H-pyrrole

-
-
71-36-3
butan-1-ol

-
A
-
3042-22-6
2-phenyl-1H-pyrrole

-
B
-
76892-15-4
N-(α-butoxyethyl)-2-phenylpyrrole

| Conditions | Yield |
|---|---|
| With ferric nitrate In water at 50℃; for 20h; Title compound not separated from byproducts; | A 10% B 85% |
| With ferric nitrate In water at 50℃; for 20h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With ferric nitrate In water; butan-1-ol at 50℃; for 20h; Product distribution; | A 10 % Chromat. B 85 % Chromat. |
| ferric nitrate In water at 50℃; for 20h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
1616603-33-8
C13H15NO2

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With copper(I) bromide In toluene at 120℃; Inert atmosphere; | 84% |
-
-
1616603-34-9
C15H19NO2

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With copper(I) bromide In toluene at 120℃; Inert atmosphere; | 82% |
-
-
80288-27-3
(2E,4Z)-6-Azido-3-phenyl-hexa-2,4-dienoic acid ethyl ester

-
A
-
3042-22-6
2-phenyl-1H-pyrrole

-
B
-
80288-31-9
ethyl α-diazo-2-phenyl-2,5-dihydropyrrole-2-acetate

| Conditions | Yield |
|---|---|
| With acetic acid In diethyl ether for 36h; Ambient temperature; | A 80% B n/a |
| In benzene at 55℃; for 1.5h; | A n/a B 25% |

| Conditions | Yield |
|---|---|
| Stage #1: trans-2-(tributylstannyl)-N,N-dibenzylcyclopropylamine; benzonitrile With n-butyllithium In tetrahydrofuran; hexane at -30 - 0℃; for 2.5h; Stage #2: With acetic acid In tetrahydrofuran; hexane | 80% |
-
-
1616603-35-0
1-phenylbut-3-en-1-one O-benzoyl oxime

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With copper(I) bromide In toluene at 120℃; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 150℃; for 6h; | 79% |
| With potassium hydroxide In dimethyl sulfoxide at 150℃; for 4h; | 44% |
| Trofimov reaction; | |
| In dimethyl sulfoxide Alkaline conditions; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 80℃; for 10h; under air; | 78% |
| Inert atmosphere; Schlenk technique; | 74% |
| With sodium hydroxide at 80℃; for 10h; Inert atmosphere; Schlenk technique; | 70% |
| With sodium hydroxide at 80℃; Schlenk technique; Inert atmosphere; | 69% |
| With palladium diacetate; acetic acid at 20℃; for 15h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 80℃; for 10h; under air; regioselective reaction; | A 78% B n/a |

-
-
109-97-7
pyrrole

-
-
66003-76-7
Diphenyliodonium triflate

-
A
-
3042-22-6
2-phenyl-1H-pyrrole

-
B
-
591-50-4
iodobenzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 80℃; for 10h; under air; regioselective reaction; | A 78% B n/a |

-
-
109-97-7
pyrrole

-
-
1483-73-4
diphenyliodonium bromide

-
A
-
3042-22-6
2-phenyl-1H-pyrrole

-
B
-
591-50-4
iodobenzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 80℃; for 10h; under air; regioselective reaction; | A 77% B n/a |

-
-
117657-37-1
2-bromo-pyrrole-1-carboxylic acid tert-butyl ester

-
-
98-80-6
phenylboronic acid

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-pyrrole-1-carboxylic acid tert-butyl ester; phenylboronic acid With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium carbonate In ethanol; water; toluene at 110℃; for 12h; Sealed tube; Inert atmosphere; Stage #2: With potassium carbonate In methanol for 12h; Inert atmosphere; Reflux; | 77% |
-
-
161958-62-9
2-phenyl-1H-pyrrole-4-carboxylic acid

-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With ethanolamine for 2h; Heating; | 75% |

-
A
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | A n/a B 75% |

| Conditions | Yield |
|---|---|
| With C45H55O4P In dichloromethane at -65℃; for 12h; Inert atmosphere; enantioselective reaction; | 99% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
126597-34-0
1-p-hydroxyphenyl-1-phenyl-1-ethanol

| Conditions | Yield |
|---|---|
| With (11aS)-3,7-di-9-anthracenyl-10,11,12,13-tetrahydro-5-hydroxy-5-oxide diindeno[7,1de:10,70-fg][1,3,2] dioxaphosphocin In chloroform at 0 - 20℃; for 48h; Molecular sieve; enantioselective reaction; | 97% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In dichloromethane at 20℃; for 0.0333333h; | 97% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 110℃; under 735.5 Torr; for 4.5h; Product distribution; different temperature and time; | 96% |
| With potassium hydroxide In dimethyl sulfoxide at 120℃; under 735.5 Torr; for 6h; | 96% |
| With potassium hydroxide In dimethyl sulfoxide at 100℃; Product distribution; various 2-, 2,3-, and 2,5-substituted pyrroles; various times; | |
| With potassium hydroxide; benzophenone In dimethyl sulfoxide at 100℃; Product distribution; Mechanism; other pyrrole; other reagents; | |
| With potassium hydroxide; t-butylnitrite at 20℃; Mechanism; other 2-arylpyrroles; other acetylenes; |
-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
52179-74-5
5-phenyl-1H-pyrrole-2-carbaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 2-phenyl-1H-pyrrole; N,N-dimethyl-formamide With trichlorophosphate In toluene at 20℃; Vilsmeier-Haack Formylation; Cooling with ice; Inert atmosphere; Stage #2: With water; sodium hydrogencarbonate; sodium hydroxide In toluene at 0℃; for 3h; pH=7 - 12; Vilsmeier-Haack Formylation; Inert atmosphere; | 96% |
| Stage #1: 2-phenyl-1H-pyrrole; N,N-dimethyl-formamide With trichlorophosphate at 0 - 40℃; for 1.16667h; Inert atmosphere; Stage #2: With water; sodium hydroxide for 0.5h; Reflux; | |
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate In dichloromethane for 0.0833333h; Vilsmeier-Haack Formylation; Inert atmosphere; Stage #2: 2-phenyl-1H-pyrrole In dichloromethane Vilsmeier-Haack Formylation; Inert atmosphere; Stage #3: With sodium hydroxide In tetrahydrofuran at 23℃; Vilsmeier-Haack Formylation; Inert atmosphere; regioselective reaction; | 139 mg |
-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
1447004-26-3
1-(5-[1,1-biphenyl]-4-yl-1H-pyrrol-2-yl)-2,2,2-trifluoro-1-ethanol

-
-
1447031-59-5
2-[1,1-biphenyl]-4-yl-5-[2,2,2-trifluoro-1-(5-phenyl-1H-pyrrol-2-yl)-ethyl]-1H-pyrrole

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide In dichloromethane at 20℃; for 16h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-phenyl-1H-pyrrole; perfluorobenzaldehyde With trifluoroacetic acid In dichloromethane at 20℃; for 3h; Stage #2: With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane at 20℃; for 0.5h; Inert atmosphere; | 95% |
-
-
350-03-8
methyl-3-pyridylketone

-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
1149-23-1
diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate

-
-
1604834-30-1
C17H18N2O

| Conditions | Yield |
|---|---|
| With (11aR)-10,11,12,13-tetrahydro-5-hydroxy-3,7-bis[2,4,6-triisopropylphenyl]-5-oxide-diindeno[7,1-de:1',7’-fg][1,3,2]dioxaphosphocin In dichloromethane at 20℃; for 72h; Inert atmosphere; Schlenk technique; Molecular sieve; enantioselective reaction; | 94% |


| Conditions | Yield |
|---|---|
| With phosphorus pentoxide In dichloromethane at 20℃; for 16h; | 94% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
91574-67-3, 91574-68-4, 20766-37-4
2-chlorobenzalacetone

| Conditions | Yield |
|---|---|
| With 9-amino-9-deoxy-epi-cinchonidine; trifluoroacetic acid In chlorobenzene at 20℃; for 24h; Michael Addition; enantioselective reaction; | 93% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
14548-45-9
(4-bromophenyl)(pyridin-3-yl)methanone

-
-
1149-23-1
diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate

-
-
1604834-22-1
C22H19BrN2O

| Conditions | Yield |
|---|---|
| With (11aR)-10,11,12,13-tetrahydro-5-hydroxy-3,7-bis[2,4,6-triisopropylphenyl]-5-oxide-diindeno[7,1-de:1',7’-fg][1,3,2]dioxaphosphocin In dichloromethane at 20℃; for 60h; Inert atmosphere; Schlenk technique; Molecular sieve; enantioselective reaction; | 93% |

-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
64995-46-6
3-(trifluoromethyl)-2H-benzo[b][1,4]oxazin-2-one

| Conditions | Yield |
|---|---|
| With (11aR)-10,11,12,13-tetrahydro-5-hydroxy-3,7-bis[2,4,6-triisopropylphenyl]-5-oxide-diindeno[7,1-de:1',7’-fg][1,3,2]dioxaphosphocin In toluene at 20℃; for 12h; Friedel-Crafts Alkylation; enantioselective reaction; | 93% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
17784-47-3
acridine hydrochloride

| Conditions | Yield |
|---|---|
| With CdS/TiO2; oxygen In butan-1-ol at 20℃; for 5h; Irradiation; | 92% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With (R)-6,6'-bis(2,4,6-triisopropylphenyl)-1,1'-spirobiindanyl-7,7'-diyl hydrogen phosphate In toluene at 20℃; for 18h; Friedel-Crafts Alkylation; Inert atmosphere; enantioselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With 2,2,2-trifluoroacetic acid ammonia In ethanol; water at 115℃; | 91% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With (R)-6,6'-bis(2,4,6-triisopropylphenyl)-1,1'-spirobiindanyl-7,7'-diyl hydrogen phosphate In toluene at 20℃; for 18h; Friedel-Crafts Alkylation; Inert atmosphere; enantioselective reaction; | 90% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

-
-
64995-54-6
6-chloro-3-trifluoromethyl-benzoxazinone

| Conditions | Yield |
|---|---|
| With (S)-3,3'-bis(2,4,6-tri-iso-propylphenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In toluene at 20℃; for 18h; Friedel-Crafts Alkylation; Inert atmosphere; enantioselective reaction; | 90% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: 2-phenyl-1H-pyrrole With sodium hydride In N,N-dimethyl-formamide; paraffin oil at 25 - 28℃; for 0.5h; Stage #2: (E)-2-((6-chlorohex-2-en-1-yl)oxy)tetrahydro-2H-pyran In N,N-dimethyl-formamide; paraffin oil at 0 - 28℃; for 24h; | 90% |
-
-
3042-22-6
2-phenyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In dichloromethane at 20℃; for 12h; | 90% |
1H-Pyrrole, 2-phenyl- Specification
The 1H-Pyrrole, 2-phenyl-, with the CAS registry number 3042-22-6, has the systematic name of 2-phenyl-1H-pyrrole. It is also called 2-Phenylpyrrole, and its code classification is Drug/Therapeutic Agent. And the molecular formula of this chemical is C10H9N.
The physical properties of 1H-Pyrrole, 2-phenyl- are as followings: (1)ACD/LogP: 2.76; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.76; (4)ACD/LogD (pH 7.4): 2.76; (5)ACD/BCF (pH 5.5): 73.72; (6)ACD/BCF (pH 7.4): 73.72; (7)ACD/KOC (pH 5.5): 755.86; (8)ACD/KOC (pH 7.4): 755.86; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 4.93 Å2; (13)Index of Refraction: 1.596; (14)Molar Refractivity: 45.27 cm3; (15)Molar Volume: 133 cm3; (16)Polarizability: 17.94×10-24cm3; (17)Surface Tension: 42.7 dyne/cm; (18)Density: 1.076 g/cm3; (19)Flash Point: 124.6 °C; (20)Enthalpy of Vaporization: 52.09 kJ/mol; (21)Boiling Point: 302.4 °C at 760 mmHg; (22)Vapour Pressure: 0.00178 mmHg at 25°C.
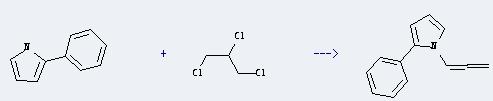
Uses of 1H-Pyrrole, 2-phenyl-: It can react with 1,2,3-trichloro-propane to produce 1-propadienyl-2-phenylpyrrole. This reaction will need reagent KOH, and the solvent dimethylsulfoxide. The reaction time is 20 minutes with temperature of 40°C, and the yield is about 2.8g.
You can still convert the following datas into molecular structure:
(1)SMILES: c2ccc(c1cccn1)cc2
(2)InChI: InChI=1/C10H9N/c1-2-5-9(6-3-1)10-7-4-8-11-10/h1-8,11H
(3)InChIKey: IRTLROCMFSDSNF-UHFFFAOYAN
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 533mg/kg (533mg/kg) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Journal of Medicinal Chemistry. Vol. 11, Pg. 1251, 1968. |
Related Products
- 1H-Pyrrole, 1-ethyl-
- 1H-Pyrrole, 1-phenyl-
- 1H-Pyrrole, 2-chloro-
- 1H-Pyrrole, 2-ethyl-
- 1H-Pyrrole, 2-phenyl-
- 1H-Pyrrole, 3-chloro-
- 1H-Pyrrole, 3-iodo-
- 1H-Pyrrole, 3-nitro-
- 1H-Pyrrole, dihydro-
- 1H-Pyrrole,1-(2-bromoethyl)-
- 3042-81-7
- 30429-79-9
- 30431-54-0
- 30431-99-3
- 30432-16-7
- 30433-78-4
- 30433-91-1
- 30437-13-9
- 30443-47-1
- 304445-49-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View