-
Name
2,2,2-Trifluoroethanol
- EINECS 200-913-6
- CAS No. 75-89-8
- Article Data132
- CAS DataBase
- Density 1.325 g/cm3
- Solubility Misible soluble with water
- Melting Point -44 °C(lit.)
- Formula C2H3F3O
- Boiling Point 74 °C at 760 mmHg
- Molecular Weight 100.04
- Flash Point 29.4 °C
- Transport Information UN 1986 3/PG 3
- Appearance colorless liquid
- Safety 26-36-39-45-36/37/39-16
- Risk Codes 10-20/21/22-37/38-41-62-38-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T,
T, F,
F, Xn
Xn
- Synonyms Trifluoroethanol;2,2,2-Trifluoroethanol, 99.8%;beta.,.beta.,.beta.-Trifluoroethyl alcohol;Fluorinol 85;2,2,2-Trifluoroethyl alcohol;beta,beta,beta-Trifluoroethyl alcohol;2,2,2,-Trifluoroethanol(TFE);2,2,2-Trifluoroethanol; TFE;2,2,2-Trifluoro Ethanol;Trifluoro Ethanol;Ethanol, 2,2,2-trifluoro-;Trifluoro Ethanol(L);Ethanol, 2,2, 2-trifluoro-;
- PSA 20.23000
- LogP 0.54100
Synthetic route

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; potassium tert-butylate; hydrogen In 1,4-dioxane at 40℃; under 7500.75 Torr; for 16h; Time; Concentration; Pressure; Temperature; Glovebox; Inert atmosphere; | 99% |
| With lithium aluminium tetrahydride; diethyl ether |

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); hydrogen; sodium methylate In methanol at 60℃; under 22502.3 Torr; for 2h; Pressure; Temperature; Time; Concentration; Reagent/catalyst; Autoclave; | 99% |
| With hydrogen; phosphan | |
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); hydrogen; sodium methylate In methanol at 40℃; under 19001.3 Torr; for 24h; Autoclave; Inert atmosphere; | 98 %Spectr. |
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); hydrogen; sodium methylate In methanol at 40℃; under 18751.9 Torr; for 24h; Catalytic behavior; Reagent/catalyst; Autoclave; | 92 %Spectr. |
| With C46H43N7OPRu(1+)*BF4(1-); potassium tert-butylate; hydrogen In 1,4-dioxane at 100℃; under 37503.8 Torr; for 2h; Inert atmosphere; Autoclave; | 100 %Spectr. |
-
-
117068-31-2
(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-n-decyl)trifluoroacetate

-
A
-
75-89-8
2,2,2-trifluoroethanol

-
B
-
678-39-7
3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-1-decanol

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A 99% B n/a |

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A 99% B n/a |
| With trimethylamine-N-oxide; tricarbonyl(η4-1,3-bis(trimethylsilyl)-4,5,6,7-tetrahydro-2H-inden-2-one)iron; hydrogen In toluene at 90℃; under 52505.3 Torr; for 17h; Catalytic behavior; Reagent/catalyst; Temperature; Inert atmosphere; Glovebox; | |
| With C21H35BrMnN2O2P; hydrogen; potassium hydride; 1,3,5-trimethyl-benzene In toluene at 100℃; under 15001.5 Torr; for 28h; Autoclave; Inert atmosphere; | A 78 %Spectr. B 99 %Spectr. |
| With C21H35BrMnN2O2P; hydrogen; potassium hydride In toluene at 100℃; under 15001.5 Torr; for 28h; | A 78 %Spectr. B 99 %Spectr. |
-
-
1524-14-7
4-methylbenzyl trifluoroacetate

-
A
-
75-89-8
2,2,2-trifluoroethanol

-
B
-
589-18-4
4-Methylbenzyl alcohol

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A 99% B n/a |
-
-
681035-98-3
4-(trifluoromethyl)benzyl trifluoroacetate

-
A
-
349-95-1
4-(trifluoromethyl)benzylic alcohol

-
B
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A n/a B 99% |
-
-
139058-95-0
4-fluorobenzyl trifluoroacetate

-
A
-
459-56-3
4-fluorobenzylic alcohol

-
B
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A n/a B 97% |
| With trimethylamine-N-oxide; tricarbonyl(η4-1,3-bis(trimethylsilyl)-4,5,6,7-tetrahydro-2H-inden-2-one)iron; hydrogen; triethylamine In toluene at 90℃; under 52505.3 Torr; for 17h; Inert atmosphere; Glovebox; |

| Conditions | Yield |
|---|---|
| With potassium γ-hydroxybutyrate In 4-butanolide at 200℃; under 11251.1 Torr; for 6h; | 96% |
| With 4-butanolide; potassium hydroxide at 200℃; under 11251.1 Torr; for 6h; | 68% |
| With potassium acetate; ethylene glycol; acetic acid |
-
-
164071-20-9
cyclohexylmethyl trifluoroacetate

-
A
-
75-89-8
2,2,2-trifluoroethanol

-
B
-
100-49-2
cyclohexylmethyl alcohol

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 48h; Glovebox; Inert atmosphere; | A 95% B n/a |
| With trimethylamine-N-oxide; tricarbonyl(η4-1,3-bis(trimethylsilyl)-4,5,6,7-tetrahydro-2H-inden-2-one)iron; hydrogen In toluene at 110℃; under 52505.3 Torr; for 17h; Catalytic behavior; Solvent; Temperature; Inert atmosphere; Glovebox; |

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A 95% B n/a |
| With trimethylamine-N-oxide; tricarbonyl(η4-1,3-bis(trimethylsilyl)-4,5,6,7-tetrahydro-2H-inden-2-one)iron; hydrogen; triethylamine In toluene at 90℃; under 52505.3 Torr; for 17h; Inert atmosphere; Glovebox; | |
| With C21H35BrMnN2O2P; hydrogen; potassium hydride; 1,3,5-trimethyl-benzene In toluene at 100℃; under 15001.5 Torr; for 60h; Autoclave; Inert atmosphere; | A 97 %Spectr. B 96 %Spectr. |
| With C21H35BrMnN2O2P; hydrogen; potassium hydride In toluene at 100℃; under 15001.5 Torr; for 60h; | A 97 %Spectr. B 96 %Spectr. |
-
-
75988-14-6
1,1,1-trifluoroethyl fluorosulfite

-
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With water at 100℃; autoclave; | 93% |
-
-
431-47-0
trifluoroacetic acid-methyl ester

-
A
-
75-89-8
2,2,2-trifluoroethanol

-
B
-
431-46-9
2,2,2-trifluoro-1-methoxy-ethanol

| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); hydrogen; sodium methylate In methanol at 40℃; under 4500.45 Torr; for 19h; Pressure; Temperature; Time; Concentration; Reagent/catalyst; Autoclave; | A 7% B 93% |
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); hydrogen; sodium methylate In methanol at 40℃; under 15201 Torr; for 6h; Autoclave; Inert atmosphere; | |
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); hydrogen; sodium methylate In methanol at 40℃; under 7600.51 Torr; for 6h; Autoclave; Inert atmosphere; |
-
-
370-69-4
tris(2,2,2-trifluoroethyl)phosphite

-
-
2648-47-7
2,2,3,3,4,4,5,5-octafluorovaleraldehyde

-
A
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| In benzene at 20 - 25℃; for 2h; | A n/a B 89% |


| Conditions | Yield |
|---|---|
| With hydrogen; <(Ph3P)(Ph2P)RuH2-K+*diglyme>2 In toluene at 90℃; under 4650.4 Torr; for 20h; Title compound not separated from byproducts; | A n/a B 88% |
| With (K(1+))2<(Ph3P)3(Ph2P)Ru2H4>(2-)*2C6H14O3; hydrogen In toluene at 90℃; under 4650.4 Torr; for 20h; |

| Conditions | Yield |
|---|---|
| With [Zn(BH4)2(py)] In tetrahydrofuran for 1h; Heating; | 85% |
| With lithium aluminium tetrahydride; diethyl ether | |
| With platinum Hydrogenation.unter Druck und anschliessenden Behandeln mit Eiswasser; |
-
-
115981-45-8
methyl(2,2,2-trifluoro-ethoxo){1,2-bis(diphenylphosphino)ethane}palladium

-
A
-
34557-54-5
methane

-
B
-
74-84-0
ethane

-
C
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| In further solvent(s) Thermolysis (120°C) of starting complex (diphenylmethane, vac.).; GLC.; | A 11% B 10% C 84% |

-
-
6864-58-0
but-2-en-1-yl 2,2,2-trifluoroacetate

-
A
-
6117-91-5
(E/Z)-2-buten-1-ol

-
B
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A n/a B 84% |
-
-
41017-81-6
2-methoxyethyl trifluoroacetate

-
A
-
109-86-4
2-methoxy-ethanol

-
B
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A n/a B 80% |
-
-
55419-66-4
trifluoroacetic acid 2-phenylethyl ester

-
A
-
75-89-8
2,2,2-trifluoroethanol

-
B
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Glovebox; Inert atmosphere; | A 78% B n/a |
| With trimethylamine-N-oxide; tricarbonyl(η4-1,3-bis(trimethylsilyl)-4,5,6,7-tetrahydro-2H-inden-2-one)iron; hydrogen In toluene at 90℃; under 52505.3 Torr; for 17h; Inert atmosphere; Glovebox; |

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 60h; Glovebox; Inert atmosphere; | A 77% B n/a |
| With trimethylamine-N-oxide; tricarbonyl(η4-1,3-bis(trimethylsilyl)-4,5,6,7-tetrahydro-2H-inden-2-one)iron; hydrogen; triethylamine In toluene at 90℃; under 52505.3 Torr; for 17h; Inert atmosphere; Glovebox; |

| Conditions | Yield |
|---|---|
| With trans-[(2,6-bis(di-tert-butylphosphinomethyl)pyridine)Fe(H)2(CO)]; hydrogen; sodium methylate In 1,4-dioxane at 40℃; under 18751.9 Torr; for 16h; Reagent/catalyst; Solvent; Time; Temperature; Glovebox; Inert atmosphere; | A 77% B n/a |
-
-
1118-45-2
2,2,2-Trifluoroethyl cyanate

-
-
1215-52-7
N-benzoyl-N'-benzylhydrazine

-
A
-
75-89-8
2,2,2-trifluoroethanol

-
B
-
27643-04-5
3-Benzyl-5-phenyl-Δ4-1,3,4-oxadiazolin-2-imin

| Conditions | Yield |
|---|---|
| In diethyl ether; ethanol for 1h; Ambient temperature; | A n/a B 75% |

-
-
182255-86-3
1-[tris(trimethylsilyl)silyl]-2,2,2-trifluoroethanol

-
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With methanol In dichloromethane at 10℃; for 0.5h; Product distribution; Irradiation; | 62% |
| In methanol; dichloromethane desilylation; Photolysis; | 62% |

| Conditions | Yield |
|---|---|
| With sulfur tetrafluoride at 160℃; for 8h; autoclave; | A 30% B 48% |
-
-
79-14-1
glycolic Acid

-
A
-
811-97-2
1,1,1,2-tetrafluoroethane

-
B
-
75-89-8
2,2,2-trifluoroethanol

-
C
-
75988-14-6
1,1,1-trifluoroethyl fluorosulfite

| Conditions | Yield |
|---|---|
| With sulfur tetrafluoride at 60℃; for 15h; autoclave; | A 26% B 5% C 42% |


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether |

-
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| beim Aufbewahren; |

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide; diethyl ether at 90℃; Hydrogenation.unter Druck; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
433-06-7
2,2,2-Trifluoroethyl p-toluenesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 18h; Inert atmosphere; | 100% |
| With triethylamine In dichloromethane at 20℃; for 18h; | 100% |
| With triethylamine In chloroform for 6h; Ambient temperature; | 98% |
-
-
64642-21-3
3-benzyl-1-methyl-2-oxo-2-phenyl-2λ5-[1,3,2]diazaphospholidin-4-one

-
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 72h; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
131123-36-9
α-t-Butyldimethylsilylbenzyl p-toluenesulfonate

-
-
134644-20-5
tert-Butyl-dimethyl-[phenyl-(2,2,2-trifluoro-ethoxy)-methyl]-silane

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
89618-93-9
2-(4-methylphenyl)prop-2-en-1-yl 4-toluenesulfonate

-
-
89619-18-1
2-(4-methylphenyl)-1-(2,2,2-trifluoroethoxy)prop-2-ene

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine at 80℃; for 24h; | 100% |
| at 65℃; Kinetics; Thermodynamic data; ΔH(excit.), ΔS(excit.), other temp.; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
78708-99-3
1-chlorocyclopropyl methyl sulfide

| Conditions | Yield |
|---|---|
| at 0℃; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
78709-02-1
1-bromocyclopropyl benzyl sulfide

| Conditions | Yield |
|---|---|
| at 0℃; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
78709-15-6
1-bromocyclopropyl phenetyl sulfide

| Conditions | Yield |
|---|---|
| at 0℃; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
100-00-5
4-chlorobenzonitrile

-
-
62149-35-3
1-(2,2,2-trifluoroethoxy)-4-nitrobenzene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; N,N-dimethyl-formamide at 25℃; for 1h; Inert atmosphere; | 100% |
| Stage #1: 2,2,2-trifluoroethanol With sodium hydride In N,N-dimethyl-formamide Cooling with liquid nitrogen; Stage #2: 4-chlorobenzonitrile In N,N-dimethyl-formamide at 100℃; for 3h; Cooling with liquid nitrogen; | 98% |
| With sodium hydride In N,N-dimethyl-formamide 1.) r.t., 2.) 130 deg C, 10 h; | 92% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
1885-14-9
phenyl chloroformate

-
-
152899-11-1
2,2,2-trifluoroethyl phenyl carbonate

| Conditions | Yield |
|---|---|
| With pyridine | 100% |
| With pyridine In dichloromethane at 20℃; for 1h; |

| Conditions | Yield |
|---|---|
| With pyridine; di-tert-butyl dicarbonate In tetrahydrofuran at 20℃; for 22h; | 100% |
| With Candida antartica lipase B at 50℃; for 16h; Molecular sieve; Enzymatic reaction; | 96% |
| With dmap; triethylamine; dicyclohexyl-carbodiimide In butan-1-ol at 20℃; pH=8 - 9; | 63.9% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
81028-03-7
(Z)-4-benzyloxy-but-2-en-1-ol

-
-
373393-05-6
[2-(2,2,2-trifluoro-ethoxy)-but-3-enyloxymethyl]-benzene

| Conditions | Yield |
|---|---|
| With tributylphosphine; 1,1'-azodicarbonyl-dipiperidine In benzene at 20℃; for 6h; Mitsunobu reaction; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
1521-51-3
rac-3-bromocyclohexene

-
-
373393-06-7
3-(2',2',2'-trifluoroethoxy)-cyclohex-1-ene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate; tetra-(n-butyl)ammonium iodide | 100% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate; tetra-(n-butyl)ammonium iodide at 20℃; for 12h; | 71% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
74407-77-5
2-methyl-3-(2',2',2'-trifluoroethoxy)-prop-1-ene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate; tetra-(n-butyl)ammonium iodide | 100% |
| Stage #1: 2,2,2-trifluoroethanol With potassium hydroxide at 40℃; Stage #2: 3-Chloro-2-methylpropene In water at 40℃; | 78% |
| With potassium carbonate In N,N-dimethyl-formamide |
-
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With iodine; potassium carbonate at 50℃; for 5h; | 100% |

| Conditions | Yield |
|---|---|
| With iodine; potassium carbonate at 50℃; for 5h; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
80885-30-9, 81028-03-7, 69152-88-1
(E)-4-(benzyloxy)-2-buten-1-ol

-
-
803730-98-5
4-(2',2',2'-trifluoroethoxy)-1-benzyloxy-but-2Z-ene

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-(benzyloxy)-2-buten-1-ol With tributylphosphine; 1,1'-azodicarbonyl-dipiperidine In benzene at 20℃; for 0.166667h; Stage #2: 2,2,2-trifluoroethanol In benzene at 20℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| With boron trifluoride; toluene at 0℃; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
27006-76-4
5-chloro-4-formyl-1,3-dimethylpyrazole

-
-
126764-32-7
1,3-dimethyl-5-(2,2,2-trifluoroethoxy)-1H-pyrazole-4-carbaldehyde

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| silver trifluoromethanesulfonate at 25℃; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
113689-58-0
bis(2-fluoro-2,2-dinitroethyl)dichloroformal

-
-
210754-61-3
bis(2-fluoro-2,2-dinitroethyl)bis(2,2,2-trifluoroethyl)orthocarbonate

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
252947-89-0
tert-butyl 2-(2,2,2-trifluoroethoxy)acetate

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroethanol With sodium hydride In tetrahydrofuran at 0 - 20℃; for 0.75h; Stage #2: bromoacetic acid tert-butyl ester In tetrahydrofuran at 20℃; for 15h; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
1026530-53-9
1,4-bis<(1-imidazolyl)carbonyloxy>-cis-2-butene

-
-
1155875-17-4
(Z)-but-2-ene-1,4-bis(2',2',2'-trifluoroethylcarbonate)

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; Inert atmosphere; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
446-35-5
2,4-Difluoronitrobenzene

-
-
186386-91-4
4-fluoro-1-nitro-2-(2,2,2-trifluoroethoxy)benzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In toluene at 30 - 50℃; for 16.5h; | 100% |
| With caesium carbonate In tetrahydrofuran at 20℃; | 99.8% |
| With caesium carbonate In tetrahydrofuran at 25℃; for 8h; | 67% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
1227410-41-4
5,11,17,23,29,35-hexa-tert-butyl-2-chloro-37,38,39,40,41,42-hexamethoxycalix[6]arene

| Conditions | Yield |
|---|---|
| In chloroform Reflux; | 100% |
-
-
17129-00-9
4-butoxy-2,2-bis-trifluoromethyl-oxetane

-
-
75-89-8
2,2,2-trifluoroethanol

-
-
1226778-13-7
C11H15F9O3

| Conditions | Yield |
|---|---|
| at 25 - 35℃; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
107-13-1
acrylonitrile

-
-
272128-06-0
3-(2,2,2-trifluoroethoxy)propionitrile

| Conditions | Yield |
|---|---|
| With [μN,κP,κC,κN-{2-(i-Pr2PO),6-(CH2NBn)-(C6H3)}Ni]2 In benzene at 20℃; for 5.5h; | 100% |
| With C31H23F3NiO5P2S; triethylamine In toluene at 60℃; for 3h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With triethylamine at 20℃; for 5h; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
14394-70-8
2-chloro-4-amino-5-methylpyrimidine

-
-
1319068-21-7
5-methyl-2-(2,2,2-trifluoro-ethoxy)-pyrimidin-4-ylamine

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroethanol With sodium for 1h; Inert atmosphere; Stage #2: 2-chloro-4-amino-5-methylpyrimidine at 90℃; | 100% |
| Stage #1: 2,2,2-trifluoroethanol With sodium at 20℃; for 1h; Inert atmosphere; Stage #2: 2-chloro-4-amino-5-methylpyrimidine at 90℃; | 100% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
400-74-8
2-Fluoro-5-nitrobenzotrifluoride

-
-
1324003-80-6
4-nitro-1-(2,2,2-trifluoroethoxy)-2-(trifluoromethyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroethanol With sodium hydride In acetonitrile; mineral oil at 20℃; for 0.166667h; Inert atmosphere; Stage #2: 2-Fluoro-5-nitrobenzotrifluoride In acetonitrile; mineral oil for 1h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 24h; Sealed tube; | 87% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
41667-95-2
5,6-dichloronicotinic acid

-
-
953729-63-0
5-chloro-6-(2,2,2-trifluoroethoxy)nicotinic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluoroethanol With sodium hydride In N,N-dimethyl acetamide; mineral oil at 0℃; for 0.333333h; Stage #2: 5,6-dichloronicotinic acid In N,N-dimethyl acetamide; mineral oil at 90℃; for 2h; | 100% |
| With potassium hydroxide In dimethyl sulfoxide at 120℃; for 24h; |
2,2,2-Trifluoroethanol Chemical Properties
Molecular Structure of 2,2,2-Trifluoroethanol (CAS NO.75-89-8):
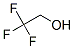
Molecular formula: C2H3F3O
Molar weight: 100.04
H bond acceptors: 1
H bond donors: 1
Freely Rotating Bonds: 1
Polar Surface Area: 20.23 Å2
Index of Refraction: 1.281
Molar Refractivity: 13.3 cm3
Molar Volume: 75.4 cm3
Surface Tension: 16.5 dyne/cm
Density: 1.325 g/cm3
Flash Point: 29.4 °C
Enthalpy of Vaporization: 36.76 kJ/mol
Boiling Point: 74 °C at 760 mmHg
Vapour Pressure: 77.3 mmHg at 25°C
EINECS: 200-913-6
Melting point: -44 °C(lit.)
Merck: 9682
BRN: 1733203
Storage temp: Store at RT
Appearance: Colorless liquid
InChI
InChI=1/C2H3F3O/c3-2(4,5)1-6/h6H,1H2
Smiles
C(CO)(F)(F)F
Stability: Stable. Flammable - note wide explosion limits. Moisture sensitive. Incompatible with strong oxidizing agents, strong acids, sodium, potassium
Product Categories: Organics; API intermediates; Other Reagents; Fluorinated Building Blocks; Fluorinating Reagents & Building Blocks for Fluorinated Biochemical Compounds; Synthetic Organic Chemistry; Chemistry
2,2,2-Trifluoroethanol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intravenous | 400mg/kg (400mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Toxicology and Applied Pharmacology. Vol. 15, Pg. 83, 1969. |
| mouse | LC50 | inhalation | 2900mg/m3 (2900mg/m3) | BRAIN AND COVERINGS: "CHANGES IN CIRCULATION (HEMORRHAGE, THROMBOSIS, ETC.)" BEHAVIORAL: GENERAL ANESTHETIC LUNGS, THORAX, OR RESPIRATION: STRUCTURAL OR FUNCTIONAL CHANGE IN TRACHEA OR BRONCHI | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 13(10), Pg. 29, 1969. |
| mouse | LD50 | intraperitoneal | 158mg/kg (158mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Annales Medicinae Experimentalis et Biologiae Fenniae. Vol. 46, Pg. 242, 1968. |
| mouse | LD50 | intravenous | 250mg/kg (250mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Annales Medicinae Experimentalis et Biologiae Fenniae. Vol. 46, Pg. 242, 1968. |
| mouse | LD50 | oral | 366mg/kg (366mg/kg) | BEHAVIORAL: TREMOR LUNGS, THORAX, OR RESPIRATION: DYSPNEA GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Toxicology and Applied Pharmacology. Vol. 15, Pg. 83, 1969. |
| rabbit | LD50 | skin | 390uL/kg (0.39mL/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | National Technical Information Service. Vol. OTS0555986, |
| rat | LC50 | inhalation | 470ppm/6H (470ppm) | BEHAVIORAL: TREMOR BEHAVIORAL: ATAXIA SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0555986, |
| rat | LD50 | intraperitoneal | 210mg/kg (210mg/kg) | Toxicology and Applied Pharmacology. Vol. 71, Pg. 84, 1983. | |
| rat | LD50 | oral | 240mg/kg (240mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 607, 1969. | |
| rat | LD50 | skin | 1680mg/kg (1680mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 607, 1969. |
2,2,2-Trifluoroethanol Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
2,2,2-Trifluoroethanol Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by inhalation and skin contact. Experimental reproductive effects. A severe skin and eye irritant. When heated to decomposition it emits toxic fumes of F−.
Hazard Codes:  Xn,
Xn, T,
T, F
F
Hazard Note: Flammable/Toxic
The Risk Statements information of 2,2,2-Trifluoroethanol (CAS NO.75-89-8):
10: Flammable
41: Risk of serious damage to eyes
62: Possible risk of impaired fertility
20/21/22: Harmful by inhalation, in contact with skin and if swallowed
37/38: Irritating to respiratory system and skin
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of 2,2,2-Trifluoroethanol (CAS NO.75-89-8):
16: Keep away from sources of ignition - No smoking
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
39: Wear eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
RIDADR: UN 1986 3/PG 3
WGK Germany: 1
RTECS: KM5250000
Hazard Note: Flammable/Toxic
HazardClass: 3
PackingGroup: III
HS Code: 29055910
2,2,2-Trifluoroethanol Specification
2,2,2-Trifluoroethanol , with CAS number of 75-89-8, can be called Trifluoroethanol ; 2,2,2-Trifluoroethanol, 99.8% ; 2,2,2-Trifluoroethyl alcohol ; beta,beta,beta-Trifluoroethyl alcohol ; Trifluoro Ethanol ; Ethanol, 2,2,2-trifluoro- ; Trifluoro Ethanol(L) ; Ethanol, 2,2, 2-trifluoro- . It is a colorless liquid. 2,2,2-Trifluoroethanol (CAS NO.75-89-8) is used as a solvent in organic chemistry. Oxidations of sulfur compounds using hydrogen peroxide are effectively conducted in TFE. In biology TFE, it is used as a co-solvent in protein folding studies with NMR spectroscopy, this solvent can effectively solubilize both peptides and proteins.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 75898-26-9
- 75898-35-0
- 75898-90-7
- 75899-68-2
- 75-90-1
- 75903-62-7
- 759-05-7
- 75907-82-3
- 75908-73-5
- 75908-83-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View