-
Name
2,5-Dichlorobenzoic acid
- EINECS 200-065-7
- CAS No. 50-79-3
- Article Data40
- CAS DataBase
- Density 1.518 g/cm3
- Solubility <0.1 g/100 mL at 19 °C in water
- Melting Point 151-154 °C(lit.)
- Formula C7H4Cl2O2
- Boiling Point 301 °C at 760 mmHg
- Molecular Weight 191.014
- Flash Point 135.8 °C
- Transport Information
- Appearance white crystalline powder
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,5-Dichlorobenzoate;2, 5-Dichlorobenzoic acid;Benzoic acid, 2,5-dichloro-;
- PSA 37.30000
- LogP 2.69160
Synthetic route

| Conditions | Yield |
|---|---|
| With cobalt(II) naphthenate; Diisobutyl adipate at 78℃; for 2h; Temperature; | 97% |

| Conditions | Yield |
|---|---|
| With water; bromine; sodium hydroxide at 0 - 20℃; for 2h; | 78.9% |
-
-
80959-18-8
2,4-dichlorobenzaldoxime

-
A
-
874-42-0
2,4-dichlorobenzaldeyhde

-
B
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With calcium hypochlorite; montmorillonite K-10 In chloroform at 20℃; for 3.3h; | A 68% B 2.3% |

| Conditions | Yield |
|---|---|
| With water; palladium diacetate; triethylamine; triphenylphosphine In 1,4-dioxane at 110℃; under 11251.1 Torr; for 2h; Flow reactor; | 48% |
-
-
95-94-3
1,2,4,5-tetrachlorobenzene

-
-
124-38-9
carbon dioxide

-
A
-
50-82-8
2,4,5-trichlorobenzoic acid

-
B
-
108-90-7
chlorobenzene

-
C
-
71-43-2
benzene

-
D
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In N,N-dimethyl-formamide at -5 - 0℃; Dehalogenation; Carboxylation; Electrochemical reaction; Further byproducts given; | A 14% B 4% C 2% D 6% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium chlorate | |
| With chloroperoxidase; dihydrogen peroxide In water pH=3.5; Chlorination; | 0.2% |

| Conditions | Yield |
|---|---|
| ueber das Nitril durch Behandeln mit alkoh. Kalilauge; | |
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid / Diazotization.Behandlung der Loesung des Diazoniumsulfats mit Kaliumkupfercyanuer 2: fuming hydrochloric acid / 180 °C View Scheme |
-
-
6361-23-5
2,5-dichloro-benzaldehyde

-
A
-
34145-05-6
2,5-dichlorobenzyl alcohol

-
B
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide | |
| With sodium amalgam |
-
-
19398-61-9
2,5-dichlorotoluene

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate | |
| With nitric acid at 140℃; im Druckrohr; |
-
-
21663-61-6
2,5-dichlorobenzonitrile

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 180℃; | |
| With potassium hydroxide In ethylene glycol at 170℃; for 7h; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride Diazotization; |
-
-
54484-63-8
2,5-dichloroethylbenzene

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With dichromate mixture |

| Conditions | Yield |
|---|---|
| With chlorine; iron(III) chloride at 35℃; nachfolgend Verseifen; |

| Conditions | Yield |
|---|---|
| With chlorine; iron(III) chloride |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium dichromate at 180℃; im Druckrohr; | |
| With chlorosulphuric acid; sulfur at 35 - 40℃; Einleiten von Chlor; |
-
-
124-38-9
carbon dioxide

-
-
120-82-1
1,2,4-Trichlorobenzene

-
A
-
50-84-0
2,4 dichlorobenzoic acid

-
B
-
51-44-5
3,4-dichlorbenzoic acid

-
C
-
95-50-1
1,2-dichloro-benzene

-
D
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In N,N-dimethyl-formamide at 5℃; electrolysis (I=0.4 A); Yield given. Further byproducts given. Yields of byproduct given; |

-
-
7647-01-0
hydrogenchloride

-
-
91532-29-5
6-nitrobenzo[d][1,2,3]triazin-4(3H)-one

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| at 150 - 160℃; |
-
-
7647-01-0
hydrogenchloride

-
-
635-21-2
5-chloroanthranilic acid

-
A
-
321-14-2
5-chloro-2-hydroxybenzoic acid

-
B
-
54389-65-0
4,4'-dichlorobiphenyl-2,2'-dicarboxylic acid

-
C
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Erhitzen der Reaktionsloesung mit ammoniakal. wss. Kupfer(I)-sulfit-Loesung; |

-
-
7782-50-5
chlorine

-
-
65-85-0
benzoic acid

-
A
-
51-44-5
3,4-dichlorbenzoic acid

-
B
-
535-80-8
3-chlorobenzoate

-
C
-
50-79-3
2,5-dichlorobenzoic acid

-
-
7647-01-0
hydrogenchloride

-
-
65-85-0
benzoic acid

-
A
-
51-44-5
3,4-dichlorbenzoic acid

-
B
-
535-80-8
3-chlorobenzoate

-
C
-
118-91-2
ortho-chlorobenzoic acid

-
D
-
50-79-3
2,5-dichlorobenzoic acid


-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With alkaline aqueous potassium permanganate | |
| With sodium hypochlorite |

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With water |

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
56-23-5
tetrachloromethane

-
-
7446-70-0
aluminium trichloride

-
-
106-46-7
para-dichlorobenzene

-
A
-
25187-09-1
2,5,2',5'-tetrachloro-benzophenone

-
B
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Zersetzung des Produkts mit Schwefelsaeure; |


-
-
1008-89-5
2-phenylpyridine

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
1453098-85-5
(2,5-dichlorophenyl)(2-(pyridin-2-yl)phenyl)methanone

| Conditions | Yield |
|---|---|
| With palladium diacetate; trifluoroacetic anhydride at 100℃; for 16h; Schlenk technique; Sealed tube; | 98% |
-
-
16066-93-6
1-(5-amino-1H-indol-1-yl)ethan-1-one

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dichlorobenzoic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane for 0.166667h; Stage #2: 1-(5-amino-1H-indol-1-yl)ethan-1-one In dichloromethane at 20℃; for 8h; | 98% |

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
1144110-81-5
C17H12Cl2N4S2

| Conditions | Yield |
|---|---|
| With trichlorophosphate for 8h; Heating; | 97% |
-
-
56-40-6
glycine

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
133433-34-8
5-chloro-2-<(carboxymethyl)amino>-benzoic acid

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate In N,N-dimethyl-formamide at 25℃; for 0.416667h; Ullmann condensation; ultrasonic irradiation; | 96% |
| With copper; potassium carbonate In N,N-dimethyl-formamide for 3h; Heating; | 87% |
-
-
136-95-8
2-amino-benzthiazole

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
37752-70-8
2-chloro-12H-benzothiazolo<2,3-b>quinazolin-12-one

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate In N,N-dimethyl-formamide at 25℃; for 0.416667h; sonication; | 95% |
| With copper Ullmann condensation; Sonication; |
-
-
64-04-0
phenethylamine

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
1002965-80-1
5-chloro-N-phenethylanthranilic acid

| Conditions | Yield |
|---|---|
| With copper(I) oxide; copper; potassium carbonate In various solvent(s) at 130℃; for 24h; | 94% |

| Conditions | Yield |
|---|---|
| at 65 - 70℃; for 8h; Temperature; Acidic conditions; | 93.6% |
| Acidic conditions; | 73% |

| Conditions | Yield |
|---|---|
| With thionyl chloride for 3h; Reflux; Inert atmosphere; | 93% |
| With thionyl chloride; N,N-dimethyl-formamide at 50℃; for 24h; | 88% |
| With thionyl chloride |

| Conditions | Yield |
|---|---|
| With pyridine; water; potassium carbonate; copper for 0.25h; sonication; | 92% |
| With pyridine; copper; potassium carbonate In water for 2h; Heating; | 84% |
| With sodium methylate at 150℃; Eintragen des Reaktionsgemisches in Wasser; | |
| With copper(l) iodide; copper; potassium carbonate at 170℃; under 4413.05 Torr; |
-
-
123-75-1
pyrrolidine

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
77265-94-2
5-chloro-2-(1-pyrrolidinyl)benzoic acid

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate In N,N-dimethyl-formamide at 25℃; for 0.25h; Ullmann condensation; ultrasonic irradiation; | 91% |

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 6-piperazin-1-yl-pyridazine-3-carboxylic acid (3-methylbutyl)amide; 2,5-dichlorobenzoic acid With benzotriazol-1-ol; 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 20℃; for 0.25h; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dichlorobenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride at -5℃; for 0.666667h; Stage #2: glycine ethyl ester hydrochloride With N-ethyl-N,N-diisopropylamine for 0.666667h; | 89% |
| Stage #1: 2,5-dichlorobenzoic acid With benzotriazol-1-ol In dichloromethane at -10℃; for 0.166667h; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 0.5h; Stage #3: glycine ethyl ester hydrochloride Further stages; | 88.9% |
| Stage #1: 2,5-dichlorobenzoic acid With 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 0℃; for 0.666667h; Stage #2: glycine ethyl ester hydrochloride With 4-methyl-morpholine In tetrahydrofuran at 0 - 20℃; for 3h; | 87% |
-
-
21909-45-5
1-benzyl-2,3-dihydro-1H-indol-5-ylamine

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dichlorobenzoic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane for 0.166667h; Stage #2: 1-benzyl-2,3-dihydro-1H-indol-5-ylamine In dichloromethane at 20℃; for 8h; | 89% |

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; | 89% |

| Conditions | Yield |
|---|---|
| With sodium chlorite; potassium iodide at 100℃; for 24h; Schlenk technique; | 89% |

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dichlorobenzoic acid With benzotriazol-1-ol In dichloromethane at -10℃; for 0.166667h; Stage #2: With 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride In dichloromethane for 0.5h; Stage #3: amino-acetic acid methyl ester hydrochloride salt In dichloromethane at 20℃; | 88.9% |
-
-
35677-83-9, 41994-45-0, 43041-11-8, 90710-04-6
methyl (S)-pipecolinate

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
1445984-86-0
(S)-methyl 1-(2,5-dichlorobenzoyl)piperidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 20℃; | 88% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-aniline for 12h; Schlenk technique; Glovebox; Reflux; | 88% |
-
-
110-89-4
piperidine

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
77265-96-4
5-chloro-2-(1-piperidinyl)benzoic acid

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate In N,N-dimethyl-formamide at 25℃; for 0.25h; Ullmann condensation; ultrasonic irradiation; | 86% |
-
-
19099-54-8
2-isopropyliodobenzene

-
-
121-46-0
bicyclo[2.2.1]hepta-2,5-diene

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With P(p-CH3OC6H4)3; palladium diacetate; caesium carbonate In 1,4-dioxane at 130℃; for 18h; Schlenk technique; Sealed tube; Inert atmosphere; | 86% |


| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dichlorobenzoic acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 23℃; for 2h; Stage #2: dimethyl amine In dichloromethane; water; N,N-dimethyl-formamide at 0 - 23℃; for 2h; | 85% |
-
-
114-33-0
N-Methylnicotinamide

-
-
6046-93-1
copper(II) acetate monohydrate

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| In acetonitrile for 24h; | 85% |

-
-
174152-11-5
(S)-2-amino-3-(4-(trifluoromethyl)phenyl)propanoic acid methyl ester

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dichlorobenzoic acid With benzotriazol-1-ol In dichloromethane at -10℃; for 0.166667h; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 0.5h; Stage #3: (S)-2-amino-3-(4-(trifluoromethyl)phenyl)propanoic acid methyl ester Further stages; | 84.3% |
| Stage #1: 2,5-dichlorobenzoic acid With benzotriazol-1-ol In dichloromethane at -10℃; for 0.166667h; Stage #2: (S)-2-amino-3-(4-(trifluoromethyl)phenyl)propanoic acid methyl ester With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at -10 - 20℃; for 16h; | |
| Stage #1: 2,5-dichlorobenzoic acid With benzotriazol-1-ol In dichloromethane at -10℃; for 0.166667h; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 0.5h; Stage #3: (S)-2-amino-3-(4-(trifluoromethyl)phenyl)propanoic acid methyl ester Further stages; |
-
-
1401197-80-5
5-(2,6-difluorophenyl)-3-phenyl-4,5-dihydropyrazole-1-carbothioamide

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With trichlorophosphate for 5h; Reflux; | 84.1% |
-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
941072-69-1
4-(2,5-dichloro-benzoyl)-piperazine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 10 - 20℃; | 84% |

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate In N,N-dimethyl-formamide at 25℃; for 0.25h; Ullmann condensation; ultrasonic irradiation; | 83% |
| With copper; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 5h; Reflux; | 2.3 g |

| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dichlorobenzoic acid With N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0℃; Inert atmosphere; Cooling with ice; Stage #2: C17H28N4O4S*C2HF3O2 With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0℃; Inert atmosphere; Cooling with ice; | 82% |
-
-
109-89-7
diethylamine

-
-
50-79-3
2,5-dichlorobenzoic acid

-
-
77265-80-6
5-chloro-2-(diethylamino)benzoic acid

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate In N,N-dimethyl-formamide at 25℃; for 0.333333h; Ullmann condensation; ultrasonic irradiation; | 81% |

-
-
50-79-3
2,5-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,5-dichlorobenzoic acid With benzotriazol-1-ol In dichloromethane at -10℃; for 0.166667h; Stage #2: (S)-2-amino-3-methoxy-N-((R)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butyl)propenamide hydrochloride With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at -10 - 20℃; | 80.8% |
2,5-Dichlorobenzoic acid Chemical Properties
IUPAC Name: 2,5-Dichlorobenzoic acid
Synonyms of 2,5-Dichlorobenzoic acid (CAS NO.50-79-3): Benzoic acid, 2,5-dichloro-
CAS NO: 50-79-3
Molecular Formula: C7H4Cl2O2
Molecular Weight: 191.01
Molecular Structure: 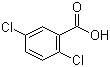
EINECS: 200-065-7
H bond acceptors: 2
H bond donors: 1
Freely Rotating Bonds: 1
Polar Surface Area: 37.3 Å2
Index of Refraction: 1.599
Molar Refractivity: 42.973 cm3
Molar Volume: 125.864 cm3
Surface Tension: 53.966 dyne/cm
Density: 1.518 g/cm3
Flash Point: 135.84 °C
Enthalpy of Vaporization: 57.14 kJ/mol
Boiling Point: 300.999 °C at 760 mmHg
Vapour Pressure: 0 mmHg at 25°C
Melting point: 152-157°C
Water solubility: <0.1 g/100 mL at 19°C
Stability: Stable.
Appearance: white crystalline powder
SMILES: O=C(O)c1cc(Cl)ccc1Cl
InChI: InChI=1/C7H4Cl2O2/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3H,(H,10,11)
InChIKey: QVTQYSFCFOGITD-UHFFFAOYAT
Std. InChI: InChI=1S/C7H4Cl2O2/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3H,(H,10,11)
Std. InChIKey: QVTQYSFCFOGITD-UHFFFAOYSA-N
Product Categories of 2,5-Dichlorobenzoic acid (CAS NO.50-79-3): Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;Phenylacetic acid;Organic acids;Acids & Esters;Chlorine Compounds;DAlphabetic;Alpha sort;D;DIA - DIC;Pesticides&Metabolites;C7;Carbonyl Compounds;Carboxylic Acids
2,5-Dichlorobenzoic acid Uses
2,5-Dichlorobenzoic acid (CAS NO.50-79-3) is used as an organic synthesis intermediate for synthetizing herbicide chloramben and dinoben.
2,5-Dichlorobenzoic acid Production
1. First, dichlorobenzene reacts with phosgene, 2,5-DICHLOROBENZOIC ACID (50-79-3) is formed from the obtained 2,5-dichloro-benzoyl chloride by hydration.
2. Paradichlorobenzol reacts with paraformaldehyde, than oxidation by potassium permanganate to form 2,5-DICHLOROBENZOIC ACID (50-79-3).
3. Use 1,2,4-trichlorobenzene as raw material to form 2,5-dichlorobenzonitrile, then 2,5-DICHLOROBENZOIC ACID (50-79-3) is synthesized from 2,5-dichlorobenzonitrile by alkaline hydrolysis.
4. 2,5-DICHLOROBENZOIC ACID (50-79-3) is synthesized from benzoyl chloride by chlorination and hydration.
5. Use paradichlorobenzene carbon tetrachloride as raw material.
2,5-Dichlorobenzoic acid Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 237mg/kg (237mg/kg) | BEHAVIORAL: REGIDITY BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | Journal of Medicinal Chemistry. Vol. 11, Pg. 1020, 1968. |
| mouse | LD50 | subcutaneous | 1200mg/kg (1200mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Biochemical Pharmacology. Vol. 13, Pg. 1538, 1964. |
2,5-Dichlorobenzoic acid Consensus Reports
Reported in EPA TSCA Inventory.
2,5-Dichlorobenzoic acid Safety Profile
Safety Information about 2,5-Dichlorobenzoic acid (CAS NO.50-79-3):
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-24/25
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S24/25: Avoid contact with skin and eyes.
S36: Wear suitable protective clothing.
WGK Germany: 3
RTECS: DG6825000
HS Code: 29163900
2,5-Dichlorobenzoic acid Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam.
Handling: Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage: Store in a cool, dry place. Store in a tightly closed container.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 50793-85-6
- 50795-82-9
- 50800-92-5
- 508-02-1
- 5080-22-8
- 50802-52-3
- 50803-29-7
- 508-04-3
- 5080-50-2
- 50807-74-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View