-
Name
2-Aminobenzimidazole
- EINECS 213-280-6
- CAS No. 934-32-7
- Article Data94
- CAS DataBase
- Density 1.367 g/cm3
- Solubility Soluble in water (Slightly).
- Melting Point 226-230 °C(lit.)
- Formula C7H7N3
- Boiling Point 368.9 °C at 760 mmHg
- Molecular Weight 133.153
- Flash Point 204.6 °C
- Transport Information
- Appearance light yellow to cream or beige powder or flakes
- Safety 26-36/37/39-22
- Risk Codes 22-36/37/38-43
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Benzimidazole,2-amino- (6CI,7CI,8CI);1H-Benzimidazol-2-ylamine;2-Amino-1H-benzimidazole;2-Benzimidazolamine;2-Benzimidazolylamine;2-Iminobenzimidazoline;NSC 27793;NSC 7628;
- PSA 54.70000
- LogP 1.72630
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; tin bis(1,2-benzenethiolate) In tetrahydrofuran; phosphate buffer at 10℃; for 0.5h; pH=10; Reduction; | 100% |
| With dibutyltin In benzene at 40℃; for 2h; other reagents; | 98% |
| With acetic acid In dichloromethane at 20℃; for 2h; Irradiation; |

| Conditions | Yield |
|---|---|
| In methanol; water at 20 - 60℃; Inert atmosphere; | 97% |
| In ethanol; water at 70℃; for 1h; | 90% |
| In methanol; water at 50℃; for 1h; | 89% |
-
-
1184-90-3
aminoiminomethanesulfonic acid

-
-
95-54-5
1,2-diamino-benzene

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 60℃; | 95% |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
95-54-5
1,2-diamino-benzene

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With N-sulfonated biguanidine modified silica coated on cobalt ferrite nanoparticle core In ethanol at 20℃; for 0.3h; Green chemistry; | 92% |

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; for 24h; | 88% |
| In water at 20℃; | 75% |

| Conditions | Yield |
|---|---|
| at 700℃; under 0.02 Torr; Gas phase; | A 85% B 86% |

| Conditions | Yield |
|---|---|
| at 700℃; under 0.02 Torr; Gas phase; | A 78% B 85% |

| Conditions | Yield |
|---|---|
| Stage #1: bromocyane; o-phenylenediamine dihydrochloride With sodium hydrogencarbonate In water; acetonitrile at 0 - 20℃; Stage #2: With sodium carbonate In water; acetonitrile | 85% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 4h; Heating; | 82% |
-
-
1438395-75-5
N-tert-butyl-1H-benzo[d]imidazol-2-amine

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 12h; Reflux; | 82% |
-
-
162976-69-4
1H-benzimidazol-2-ylcarbamic acid methyl ester

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 2h; Reflux; | 80% |
| Stage #1: 1H-benzimidazol-2-ylcarbamic acid methyl ester With sodium hydroxide In water for 2h; Reflux; Stage #2: With hydrogenchloride In water | 65% |
| With oxygen In various solvent(s) at 20℃; for 5h; pH=11; Quantum yield; Kinetics; Further Variations:; pH-values; Reagents; conc. of dissolved O2; Decomposition; Photolysis; | |
| Alkaline conditions; | |
| With water Alkaline conditions; |
-
-
60059-51-0
N-(1H-benzimidazol-2-yl)-N'-benzylidenehydrazine

-
A
-
934-32-7
1H-benzimidazol-2-amine

-
B
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| at 700℃; under 0.02 Torr; Gas phase; | A 73% B 78% |

| Conditions | Yield |
|---|---|
| Irradiation; microwave; | 75% |

| Conditions | Yield |
|---|---|
| at 700℃; under 0.02 Torr; Gas phase; | A 65% B 67% |

| Conditions | Yield |
|---|---|
| at 700℃; under 0.02 Torr; Gas phase; | A 65% B 10% C 58% |

-
-
34840-26-1
1,3-dicarbethoxy-S-methylisothiourea

-
-
95-54-5
1,2-diamino-benzene

-
A
-
934-32-7
1H-benzimidazol-2-amine

-
B
-
615-16-7
1H-benzimidazol-2-ol

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 5h; Heating; | A 60% B 22% |

-
-
95-54-5
1,2-diamino-benzene

-
-
104619-51-4
di(1H-imidazol-1-yl)methanimine

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 12h; Heating; | 59% |
-
-
55305-43-6
N-cyano-N-phenyl-p-toluenesulfonamide

-
-
95-54-5
1,2-diamino-benzene

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran; hexane at 5 - 20℃; for 1h; Green chemistry; | 56% |
-
-
122128-68-1
2-Amino-1-benzylideneaminobenzimidazole

-
A
-
934-32-7
1H-benzimidazol-2-amine

-
B
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| In nitrobenzene for 12h; Heating; | A 47% B n/a |
-
-
60059-51-0
N-(1H-benzimidazol-2-yl)-N'-benzylidenehydrazine

-
A
-
934-32-7
1H-benzimidazol-2-amine

-
C
-
1233945-59-9
5,11-diphenyl-6H,12H-dibenzimidazo[1,2-a;1',2'-d]pyrazine

-
D
-
484-47-9
lophine

-
E
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| at 280℃; under 0.045 Torr; for 0.25h; Gas phase; | A 20% B 15% C 18% D 21% E 15% |

-
-
40828-54-4
1H-benzimidazole-2-sulfonic acid

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 145 - 150℃; for 5h; | |
| With ammonium hydroxide | |
| With ammonia |

| Conditions | Yield |
|---|---|
| With formamide Heating; Yield given. Yields of byproduct given; | |
| With formamide Heating; Yield given. Yields of byproduct given; | |
| With formamide Product distribution; Heating; also 1-alkyl-substituted benzimidazole-2-sulfonic acids investigated; |

| Conditions | Yield |
|---|---|
| With bromine 1) water, 5 deg C; 2) water, room temperature, 5 h.; Yield given. Multistep reaction; |
-
-
245111-10-8
2-(1-ethylbenzimidazol-2-yl)methyleneaminobenzimidazole

-
A
-
934-32-7
1H-benzimidazol-2-amine

-
B
-
34734-20-8
1-ethyl-1H-benzo[d]imidazole-2-carbaldehyde

| Conditions | Yield |
|---|---|
| With water Hydrolysis; |

-
A
-
934-32-7
1H-benzimidazol-2-amine

-
B
-
118482-08-9
1-vinylbenzimidazole-2-carboxaldehyde

| Conditions | Yield |
|---|---|
| With water In dimethylsulfoxide-d6 for 120h; Hydrolysis; |

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
934-32-7
1H-benzimidazol-2-amine

-
-
22945-27-3
cyclohexanone-2-carboxamide

-
-
83785-99-3
1,2,3,4,6,12-Hexahydrobenzimidazo<2,1-b>chinazolin-12-on

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 3h; Heating; | 100% |
-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| In ethanol Reflux; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Heating; | 99% |
| With triethylamine for 16h; Cooling with ice; | 72% |
| With triethylamine at 50 - 55℃; |
-
-
289499-65-6
3-(2-furyl)-2-(2-methoxycarbonylphenylhydrazono)-3-oxo-propanal

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| In ethanol for 0.166667h; microwave irradiation; | 99% |
-
-
289499-66-7
2-(2-methoxycarbonylphenylhydrazono)-3-oxo-3-(2-pyrrolyl)propanal

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| In ethanol for 0.166667h; microwave irradiation; | 99% |
-
-
934-32-7
1H-benzimidazol-2-amine

-
-
83-72-7
2-Hydroxy-1,4-naphthoquinone

-
-
555-16-8
4-nitrobenzaldehdye

-
-
1259091-97-8
2-(((1H-benzo[d]imidazol-2-yl)amino)(4-nitrophenyl)methyl)-3-hydroxynaphthalene-1,4-dione

| Conditions | Yield |
|---|---|
| With MCM-41 In ethanol at 20℃; for 1.16667h; Green chemistry; | 99% |
| With montmorillonite K-10 In ethanol at 20℃; for 8h; | 93% |
| With indium(III) chloride In water for 6h; Reflux; | 80% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
61066-33-9, 61066-34-0, 61066-35-1, 61127-23-9
pyrimidine-2,4,5,6(1H,3H)-tetraone

-
-
615-94-1
2,5-dihydroxy-1,4-benzoquinone

-
-
1367748-62-6
2-amino-1H-benzo[d]imidazol-3-ium 3-hydroxy-1-(5-hydroxy-2,4,6-trioxohexahydropyrimidin-5-yl)-2,5,6-trioxocyclohex-3-en-1-ide

| Conditions | Yield |
|---|---|
| In chloroform for 4h; Reflux; | 99% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
459-57-4
4-fluorobenzaldehyde

-
-
107-91-5
cyanoacetic acid amide

-
-
1392308-35-8
2,5-bis(4-fluorophenyl)-2,3,5,12-tetrahydrobenzo[4,5]imidazo[1,2-a]pyrimido[4,5-d]pyrimidin-4(1H)-one

| Conditions | Yield |
|---|---|
| With silica sulfuric acid In ethylene glycol at 120℃; for 0.0166667h; Catalytic behavior; Reagent/catalyst; Solvent; Green chemistry; | 99% |
| With poly(ethylene glycol)-400 at 20℃; for 1h; Green chemistry; | 90% |
| In water for 2h; Reflux; | 89% |


| Conditions | Yield |
|---|---|
| With acetic acid at 60℃; for 0.333333h; Green chemistry; | 99% |


| Conditions | Yield |
|---|---|
| With silica sulfuric acid In ethylene glycol at 120℃; for 0.0166667h; Green chemistry; | 99% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
69954-01-4
2,3,5-trichloro-6-methoxybenzoic acid chloride

| Conditions | Yield |
|---|---|
| With triethylamine In acetone at 55℃; | 98.7% |
| 98% |
-
-
934-32-7
1H-benzimidazol-2-amine

-
-
99-61-6
3-nitro-benzaldehyde

-
-
124066-54-2
(1H-benzimidazol-2-yl)-(3-nitro-benzyliden)-amine

| Conditions | Yield |
|---|---|
| In toluene for 20h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With silica In acetonitrile at 25 - 30℃; for 0.5h; Green chemistry; | 98% |
| With toluene-4-sulfonic acid In acetonitrile at 40 - 50℃; for 0.333333h; Green chemistry; | 98% |
| With starch functionalized Fe3O4 nanoparticles In water at 20℃; for 0.116667h; Irradiation; Green chemistry; chemoselective reaction; | 98% |


| Conditions | Yield |
|---|---|
| With starch functionalized Fe3O4 nanoparticles In water at 20℃; for 0.15h; Irradiation; Green chemistry; chemoselective reaction; | 98% |
| With acetic acid at 60℃; for 0.333333h; Green chemistry; | 96% |
| With 4-methyl-4-sulfonic acid morpholinium chloride at 90℃; for 0.0833333h; Reagent/catalyst; | 96% |


| Conditions | Yield |
|---|---|
| In water for 0.025h; microwave irradiation; | 98% |


| Conditions | Yield |
|---|---|
| With butane-1-sulfonic acid modified starch coated γ-Fe2O3 magnetic nanoparticles In neat (no solvent) at 100℃; for 0.166667h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; | 98% |
| With starch functionalized Fe3O4 nanoparticles In water at 20℃; for 0.116667h; Irradiation; Green chemistry; chemoselective reaction; | 98% |
| With 4-methyl-4-sulfonic acid morpholinium chloride at 90℃; for 0.0666667h; Catalytic behavior; Reagent/catalyst; Temperature; | 97% |

-
-
824953-65-3
3-(4-chlorophenyl)-2-(2-methoxycarbonylphenylhydrazono)-3-oxo-propanal

-
-
934-32-7
1H-benzimidazol-2-amine

| Conditions | Yield |
|---|---|
| In ethanol for 0.166667h; microwave irradiation; | 98% |
-
-
934-32-7
1H-benzimidazol-2-amine

-
-
360574-95-4
2-[hydroxy-(3-nitro-phenyl)-methyl]-acrylic acid methyl ester

-
-
1227670-24-7
6-[(3-nitrophenyl)hydroxymethyl]-5,6-dihydro-8H-benzo[4,5]imidazo[1,2-a]pyrimidin-7-one

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 100℃; for 2h; | 98% |
| In tetrahydrofuran; water at 50℃; for 24h; Michael addition-cyclization tandem reaction; | 77% |
-
-
872-85-5
pyridine-4-carbaldehyde

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
141-97-9
ethyl acetoacetate

-
-
727405-62-1
ethyl 2-methyl-4-(pyridin-4-yl)-1,4-dihydrobenzo[4,5]imidazo[1,2-a]pyrimidine-3-carboxylate

| Conditions | Yield |
|---|---|
| With Thiamine hydrochloride In water for 3h; Reflux; | 98% |
| With zinc perchlorate In methanol for 6h; Reflux; | 70% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
141-97-9
ethyl acetoacetate

-
-
587-04-2
3-Chlorobenzaldehyde

-
-
727405-73-4
4-(3-chlorophenyl)-2-methyl-1,4-dihydrobenzo[4,5]imidazo[1,2-a]pyrimidine-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With poly(vinylpyrrolidonium) perchlorate ([PVPH]ClO4) at 100℃; for 0.333333h; | 98% |
| With N,N,N’,N’-tetrabromobenzene-1,3-disulfonamide In neat (no solvent) at 100℃; for 0.666667h; Reagent/catalyst; Green chemistry; | 96% |
| With 4,4'-(butane-1,4-diyl)bis(1-sulfo-1,4-diazabicyclo[2.2.2]octane-1,4-diium) tetrachloride In neat (no solvent) at 90℃; for 0.25h; Green chemistry; | 92% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
541540-90-3
2,5-bis(chloromethyl)-1,3,4-oxadiazole

-
-
1489236-38-5
C18H16N8O

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetone Reflux; | 98% |
-
-
934-32-7
1H-benzimidazol-2-amine

-
-
83124-74-7
4-ethoxy-1,1,1-trichloro-3-buten-2-one

-
-
1320212-46-1
2-(trichloromethyl)benzo[4,5]imidazol[1,2-a]pyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 2h; Reflux; | 98% |
-
-
934-32-7
1H-benzimidazol-2-amine

-
-
104-88-1
4-chlorobenzaldehyde

-
-
109-77-3
malononitrile

-
-
378760-56-6
2-amino-4-(4-chlorophenyl)-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With Ag-TiO2 nanocomposite In neat (no solvent) at 60℃; for 0.133333h; Catalytic behavior; Solvent; Temperature; | 98% |
| With starch functionalized Fe3O4 nanoparticles In water at 20℃; for 0.05h; Irradiation; Green chemistry; chemoselective reaction; | 97% |
| With poly(vinylpyrrolidonium) perchlorate ([PVPH]ClO4) at 100℃; for 0.25h; | 96% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
459-57-4
4-fluorobenzaldehyde

-
-
109-77-3
malononitrile

-
-
380891-82-7
2-amino-4-(4-fluorophenyl)-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With starch functionalized Fe3O4 nanoparticles In water at 20℃; for 0.05h; Irradiation; Green chemistry; | 98% |
| With Ag-TiO2 nanocomposite In neat (no solvent) at 60℃; for 0.133333h; | 97% |
| With zinc ferrite In methanol at 70℃; for 1.48333h; Sonication; Green chemistry; | 92% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
109-77-3
malononitrile

-
-
933194-36-6
2-amino-4-(3-bromophenyl)-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With poly(vinylpyrrolidonium) perchlorate ([PVPH]ClO4) at 100℃; for 0.0666667h; | 98% |
| With 1-methyl-3-(trimethoxysilylpropyl)imidazolium hydrogen sulfate supported on rice husk ash In neat (no solvent) at 100℃; for 0.05h; Green chemistry; | 90% |
| With zinc ferrite In methanol at 70℃; for 1.36667h; Sonication; Green chemistry; | 90% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
122-03-2
(4-isopropylbenzaldehyde)

-
-
109-77-3
malononitrile

-
-
932995-93-2
2-amino-4-(4-isopropylphenyl)-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With poly(vinylpyrrolidonium) perchlorate ([PVPH]ClO4) at 100℃; for 0.0666667h; | 98% |
| With toluene-4-sulfonic acid In neat (no solvent) at 80℃; for 0.55h; Green chemistry; | 92% |
| With 1-methyl-3-(trimethoxysilylpropyl)imidazolium hydrogen sulfate supported on rice husk ash In neat (no solvent) at 100℃; for 0.0833333h; Green chemistry; | 88% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
109-77-3
malononitrile

-
-
1608468-74-1
2-amino-4-(4-bromophenyl)-1,4-dihydrobenzo[4,5]imidazolo[1,2-a]pyrimidine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With Ag-TiO2 nanocomposite In neat (no solvent) at 60℃; for 0.166667h; | 98% |
| With starch functionalized Fe3O4 nanoparticles In water at 20℃; for 0.0666667h; Irradiation; Green chemistry; | 97% |
| With poly(vinylpyrrolidonium) perchlorate ([PVPH]ClO4) at 100℃; for 0.333333h; | 95% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
141-97-9
ethyl acetoacetate

-
-
932210-11-2
ethyl 4-(3-bromophenyl)-1,4-dihydro-2-methylpyrimido[1,2-a]benzimidazole-3-carboxylate

| Conditions | Yield |
|---|---|
| With poly(vinylpyrrolidonium) perchlorate ([PVPH]ClO4) at 100℃; for 0.666667h; | 98% |
| With Copper-polysulfonamide complex immobilized on graphene oxide In ethanol for 0.333333h; Reflux; | 95% |
| With N,N,N’,N’-tetrabromobenzene-1,3-disulfonamide In neat (no solvent) at 100℃; for 0.583333h; Reagent/catalyst; Green chemistry; | 90% |

-
-
934-32-7
1H-benzimidazol-2-amine

-
-
1622068-87-4
3-[3-(2-oxiranyl-ethoxy)-6-oxo-6H-pyridazin-1-ylmethyl]-benzoic acid methyl ester

-
-
1622069-40-2
potassium 3-{3-[4-(2-amino-benzoimidazol-1-yl)-3-hydroxy-butoxy]-6-oxo-6H-pyridazin-1-ylmethyl}-benzoate

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water at 100℃; for 1h; Microwave irradiation; | 98% |
2-AMINOBENZIMIDAZOLE Chemical Properties
The Molecular Weight of 2-AMINOBENZIMIDAZOLE(934-32-7): 133.15
Molecular Structure:
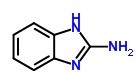
EINECS: 213-280-6
Melting point: 226-230 °C(lit.)
Boiling Point: 368.9 °C at 760 mmHg
Flash Point: 204.6 °C
Index of Refraction: 1.78
Molar Refractivity: 40.85 cm3
Molar Volume: 97.3 cm3
Polarizability: 16.19 10-24 cm3
Surface Tension: 79.4 dyne/cm
Density: 1.367 g/cm3
Enthalpy of Vaporization: 61.56 kJ/mol
Vapour Pressure: 1.23E-05 mmHg at 25°C
IUPAC Name: 1H-benzimidazol-2-amine
Synonyms: 2-Iminobenzimidazoline;Benzimidazole, 2-amino-;2-amino-benzimidazol;USAF ek-4037;usafek-4037;2-BENZIMIDAZOLAMINE;2-BENZIMIDAZOLYLAMINE;2-AMINOBENZIMIDAZOLE;
2-AMINOBENZIMIDAZOLE Toxicity Data With Reference
| 1. | mma-sat 710 µmol/L | ENMUDM Environmental Mutagenesis. 3 (1981),11. | ||
| 2. | mmo-sat 100 µg/plate | MUREAV Mutation Research. 15 (1972),273. | ||
| 3. | orl-rat TDLo:426 mg/kg (8-15D preg):TER | THERAP Therapie. 31 (1976),505. | ||
| 4. | orl-rat LDLo:500 mg/kg | NCNSA6 National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. 5 (1953),22. | ||
| 5. | orl-mus LD40:600 mg/kg | JACSAT Journal of the American Chemical Society. 67 (1945),905. | ||
| 6. | ipr-mus LD50:100 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . | ||
| 7. | ivn-mus LD50:126 mg/kg | 29QHAQ Principles of Medicinal Chemistry Foye, W.O., eds.,Philadelphia, PA.: Lea and Febiger,1974,246. |
2-AMINOBENZIMIDAZOLE Consensus Reports
2-AMINOBENZIMIDAZOLE Safety Profile
The Hazard Codes of 2-AMINOBENZIMIDAZOLE(934-32-7):
 Xn
Xn The Risk Statements information of 2-AMINOBENZIMIDAZOLE(934-32-7):
22: Harmful if swallowed
43: May cause sensitization by skin contact
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of 2-AMINOBENZIMIDAZOLE(934-32-7):
22: Do not breathe dust
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
WGK Germany: 3
RTECS: DD5775000
HS Code: 29339990
Related Products
- 2-AMINOBENZIMIDAZOLE
- 934-34-9
- 934-35-0
- 93435-21-3
- 934353-76-1
- 934-37-2
- 93438-37-0
- 934384-85-7
- 93438-65-4
- 934389-88-5
- 934405-34-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View