-
Name
2-Allylphenol
- EINECS 217-119-0
- CAS No. 1745-81-9
- Article Data148
- CAS DataBase
- Density 1.014 g/cm3
- Solubility 7 g/L (20 °C) in water
- Melting Point -6 °C
- Formula C9H10O
- Boiling Point 219.999 °C at 760 mmHg
- Molecular Weight 134.178
- Flash Point 88.889 °C
- Transport Information UN 2923 8/PG 3
- Appearance clear colorless to light yellow liquid.
- Safety 26-36/37/39-45-24/25-23
- Risk Codes 21/22-34-36/38
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, C
C
- Synonyms Phenol,2-(2-propenyl)- (9CI);Phenol, o-allyl- (6CI,8CI);2-(2-Propenyl)phenol;o-(2-Propenyl)phenol;o-Allylphenol;
- PSA 20.23000
- LogP 2.12070
Synthetic route

| Conditions | Yield |
|---|---|
| In toluene at 250℃; for 1.75h; Claisen rearrangement; microwave irradiation; | 100% |
| In chlorobenzene at 250℃; for 1.75h; Claisen rearrangement; microwave irradiation; | 100% |
| In N,N-dimethyl-aniline at 250℃; for 1h; Claisen Rearrangement; Inert atmosphere; Microwave irradiation; Sealed tube; | 100% |
-
-
18042-43-8
2-allyl-1-(trimethylsiloxy)benzene

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With methanol; 1,3-disulfonic acid imidazolium hydrogen sulfate at 20℃; for 0.0666667h; Green chemistry; | 98% |
| With methanol at 20℃; for 0.333333h; | 85% |
-
-
59152-49-7
2-(iodomethyl)-2,3-dihydro-1-benzofuran

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With dimethylboron bromide; tetra-(n-butyl)ammonium iodide; triethylamine In dichloromethane at 0℃; for 12h; | 92% |

| Conditions | Yield |
|---|---|
| In water | 87% |
-
-
1354644-30-6
N-(2-allyloxybenzylidene)-2-phenylthiobenzenamine

-
A
-
92-52-4
biphenyl

-
B
-
3411-95-8
2-(benzothiazol-2-yl)phenol

-
C
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| at 150 - 650℃; under 0.013 Torr; for 0.25h; Pyrolysis; | A 2% B 84% C 9% |


| Conditions | Yield |
|---|---|
| With aluminium(III) iodide; diisopropyl-carbodiimide In acetonitrile at 80℃; for 18h; | 82% |
-
-
73047-40-2
3-bromochroman

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| In acetonitrile Product distribution; electrochemical reductions of derivatives; 0.1 M Et4NClO4, -2.05 V; | 80% |
| In acetonitrile electrochemical reduction, 0.1M Et4NClO4, -2.05 V; | 80% |

| Conditions | Yield |
|---|---|
| With [Ir(1,5-cyclooctadiene)2]PF6 In octane at 120℃; for 20h; | A 80% B 8% |

-
-
151950-94-6
1-tert-butyldimethylsilyloxy-2-(2-propenyl)benzene

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With copper(ll) bromide In acetonitrile at 20℃; for 3h; | 77% |
-
-
51496-94-7
1-allyl-2-(benzyloxy)benzene

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With aluminium(III) iodide; diisopropyl-carbodiimide In acetonitrile at 80℃; for 18h; | 68% |

| Conditions | Yield |
|---|---|
| With copper; copper(II) perchlorate In diethyl ether | 65% |
| With 1.) K; zinc(II) chloride 1.) xylene, 3 h, reflux, 2.) 13 h, reflux; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With C6H10BBr3 In dichloromethane at 20℃; for 0.0833333h; Claisen rearrangement; Inert atmosphere; | A 61% B 12% |
| With aluminium(III) iodide In carbon disulfide at -70℃; for 3.5h; | A 26% B 4.5% |
| at 200℃; for 4h; Claisen rearrangement; neat (no solvent); | |
| With tetrabutylammomium bromide at 245℃; for 0.5h; Claisen Rearrangement; Microwave irradiation; |
-
-
1746-13-0
allyl phenyl ether

-
A
-
1746-11-8
2-methyl-2,3-dihydro-1-benzofuran

-
B
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With silica gel at 60℃; for 5h; | A 60% B 33% |
| With silica gel at 60℃; for 5h; | A 60% B 33% |
| With silica gel at 60℃; for 5h; Product distribution; other allylaryl ethers; variation of reaction time, also in benzene (reflux); | A 60% B 33% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-phenol With hydrogenchloride; water; isopentyl nitrite Stage #2: 3-chloroprop-1-ene With ferrous(II) sulfate heptahydrate; dimethyl sulfoxide In water for 0.416667h; | A 54% B n/a |

-
-
1746-13-0
allyl phenyl ether

-
A
-
501-92-8
4-(prop-2-enyl)phenol

-
B
-
1745-81-9
2-Allylphenol

-
C
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With β‐cyclodextrin In water at 25℃; for 0.5h; Irradiation; | A 21.9% B 40.2% C 2.1% |
| With β‐cyclodextrin In water at 25℃; for 0.5h; Quantum yield; Irradiation; without β-CD, in nitrogen or oxygen atmosphere; | A 21.9% B 40.2% C 2.1% |
| In methanol Product distribution; Kinetics; Quantum yield; Further Variations:; Solvents; Reagents; photo-Claisen rearrangement; Irradiation; | |
| In butan-1-ol at 35℃; for 0.0833333h; Temperature; Time; Concentration; Claisen Rearrangement; Flow reactor; UV-irradiation; |

-
-
1354644-33-9
N-(2-allyloxybenzylidene)-2-phenoxybenzenamine

-
A
-
833-50-1
2-phenylbenzo[d]oxazole

-
B
-
835-64-3
2-(2-Hydroxyphenyl)benzoxazole

-
C
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| at 130 - 650℃; under 0.01 Torr; for 0.416667h; Pyrolysis; | A 14% B 36% C 6% |

-
-
107-05-1
3-chloroprop-1-ene

-
-
108-95-2
phenol

-
A
-
1746-13-0
allyl phenyl ether

-
B
-
3383-05-9
allyl 2-allylphenyl ether

-
C
-
68714-32-9
1-allyl-4-(allyloxy)benzene

-
D
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; phthalocyanine VII at 180℃; for 2h; Alkylation; Further byproducts given; | A 35% B 10% C 5% D 20% |
| With potassium hydroxide; phthalocyanine III at 180℃; for 2h; Alkylation; | A 20% B 11% C 4% D 15% |

-
-
1354644-23-7
N-(2-allyloxybenzylidene)-2-methylbenzenamine

-
A
-
4749-47-7
2-(1H-indol-2-yl)phenol

-
B
-
3246-73-9
N-salicylidene-o-methylaniline

-
C
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| at 120 - 650℃; under 0.02 Torr; for 0.333333h; Pyrolysis; | A 35% B 7% C 5% |


-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With aluminium(III) iodide; diisopropyl-carbodiimide In acetonitrile at 80℃; for 18h; | 31% |
-
-
10575-90-3
N-nitroso-N-cyclopropylurea

-
-
108-95-2
phenol

-
C
-
1746-13-0
allyl phenyl ether

-
D
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With caesium carbonate In dichloromethane at 5 - 8℃; for 0.833333h; Further byproducts given; | A 17% B 12% C 5.5% D 6% |


| Conditions | Yield |
|---|---|
| With hydroquinone In various solvent(s) at 200℃; for 24h; Addition; | A 10% B 3.3% C 7% D 0.3% |


| Conditions | Yield |
|---|---|
| at 190℃; Kinetics; |

| Conditions | Yield |
|---|---|
| at 200℃; Kinetics; |
-
-
32704-22-6
2-allyl-phenylamine

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium nitrite anschliessend Erwaermen; |
-
-
59086-52-1
2-allyloxybenzoic acid

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With 2,3-Dimethylaniline |
-
-
59086-52-1
2-allyloxybenzoic acid

-
A
-
42729-96-4
2-Hydroxy-3-(2-propenyl)-benzoesaeure

-
B
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| at 170 - 235℃; | |
| at 180℃; |
-
-
42729-96-4
2-Hydroxy-3-(2-propenyl)-benzoesaeure

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With 2,3-Dimethylaniline | |
| at 300℃; |
-
-
71318-55-3
4-hydroxy-3-allyl-benzoic acid

-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With quinoline beim Erhitzen bis zum Sieden; |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol for 14h; Solvent; Reflux; | 100% |
| With ethanol; lithium; nickel dichloride; 4,4'-di-tert-butylbiphenyl In tetrahydrofuran at 20℃; for 12h; | 99% |
| With hydrogen; NiCl2-Li-[poly(2-vinyl-naphthalene)-co-(divinylbenzene)] In tetrahydrofuran at 20℃; under 760.051 Torr; for 1.5h; | 98% |

| Conditions | Yield |
|---|---|
| tris(triphenylphosphine)ruthenium(II) chloride at 100℃; for 1h; | 100% |
| With potassium tert-butylate In tetrahydrofuran at 20℃; for 12h; | 94% |
| With [1,3-Mes2-(N2C3H5)]Cl2(PCy3)Ru=CHPh In methanol at 60℃; for 3h; | 92% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
1745-81-9
2-Allylphenol

-
-
151950-94-6
1-tert-butyldimethylsilyloxy-2-(2-propenyl)benzene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 23℃; | 100% |
| With 1H-imidazole In dichloromethane at 20℃; for 18h; Inert atmosphere; | 99% |
| With 1H-imidazole In dichloromethane at 20℃; Schlenk technique; | 99% |
-
-
1745-81-9
2-Allylphenol

-
-
106-89-8
epichlorohydrin

-
-
49716-04-3
1-(2-allyl-phenoxy)-3-chloro-propan-2-ol

| Conditions | Yield |
|---|---|
| With pyridine In chloroform; water; phenol | 100% |
| With piperidine Heating; |
-
-
1314092-54-0
(2S)-glycidyl-3-nitrobenzenesulfonate

-
-
1745-81-9
2-Allylphenol

-
-
81840-59-7
(S)1-(2’-allylphenoxy)-2,3-epoxypropane

| Conditions | Yield |
|---|---|
| Stage #1: o-Allylphenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.5h; Inert atmosphere; Stage #2: (2S)-glycidyl-3-nitrobenzenesulfonate In N,N-dimethyl-formamide; mineral oil at 60℃; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 0 - 20℃; for 2h; Reagent/catalyst; Green chemistry; | 100% |
| With 1,4-diaza-bicyclo[2.2.2]octane In dichloromethane |
-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With tellurium tetrachloride In dichloromethane at 39 - 40℃; for 3h; Reflux; | 100% |
| With tellurium tetrachloride In tetrachloromethane for 6h; Reflux; | 90% |
-
-
1745-81-9
2-Allylphenol

| Conditions | Yield |
|---|---|
| With tellurium(IV) tetrabromide In acetonitrile at 20 - 25℃; for 20h; | 100% |
| With tellurium(IV) tetrabromide In acetonitrile at 20 - 25℃; for 20h; | 100% |
| With tellurium(IV) tetrabromide In acetonitrile at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With iodine at 25℃; for 0.0166667h; | 99% |
| With triethylamine at 20℃; for 25h; | 99% |
| With dmap In dichloromethane at 20℃; for 2.5h; | 96% |

| Conditions | Yield |
|---|---|
| With zeolite BEA/37.5 at 39.84℃; for 1h; Catalytic behavior; Reagent/catalyst; Time; Temperature; | 99% |
| With [Nd(acetonitrile)9][AlCl4]3*acetonitrile In chloroform at 65℃; for 24h; Reagent/catalyst; Solvent; Inert atmosphere; Schlenk technique; regioselective reaction; | 97% |
| With aluminium(III) triflate In nitromethane at 101℃; for 3h; | 93% |
-
-
105-36-2
ethyl bromoacetate

-
-
1745-81-9
2-Allylphenol

-
-
181027-13-4
2-(prop-2-enylphenoxy)-ethanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone for 16h; Heating; | 99% |
| With sodium hydride 1.) DMF; Yield given. Multistep reaction; | |
| With caesium carbonate In N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| Stage #1: o-Allylphenol With sodium hexamethyldisilazane In tetrahydrofuran for 0.5h; Stage #2: 3-chloro-2-fluoropropene In N,N-dimethyl-formamide at 20℃; for 12h; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 18h; | 99% |
-
-
1745-81-9
2-Allylphenol

-
-
24454-25-9
3-(2-hydroxyphenyl)propane-1,2-diol

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide; N-methyl-2-indolinone In water; acetone at 20℃; | 99% |
| Multi-step reaction with 3 steps 1: 92 percent / pyridine / 2 h / 100 °C 2: 73 percent / m-chloroperbenzoic acid / CH2Cl2 / 18 h / 20 °C 3: 70 percent / aq. sodium hydroxide / 0.5 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| With formic acid; palladium diacetate; triphenylphosphine In 1,3,5-trimethyl-benzene at 90℃; for 16h; | 99% |
-
-
542-69-8
1-iodo-butane

-
-
201230-82-2
carbon monoxide

-
-
1745-81-9
2-Allylphenol

-
-
35151-20-3
Valeriansaeure-<2-allyl-phenylester>

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride; 1,3-bis-(diphenylphosphino)propane; sodium carbonate; sodium bromide In 1,4-dioxane at 120℃; under 750.075 Torr; for 24h; Inert atmosphere; chemoselective reaction; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: resorcinol diglycidyl ether; o-Allylphenol With potassium methanolate at 110℃; for 1.7h; Inert atmosphere; Stage #2: 1,2-epoxytetradecane at 120℃; for 21.4333h; Inert atmosphere; | 98.1% |

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
1745-81-9
2-Allylphenol

-
-
18042-43-8
2-allyl-1-(trimethylsiloxy)benzene

| Conditions | Yield |
|---|---|
| With 3-methyl-1-sulfonic acid imidazolium hydrogen sulfate at 20℃; for 0.0333333h; Neat (no solvent); | 98% |
| With 1,3-disulfonic acid imidazolium hydrogen sulfate In neat (no solvent) at 20℃; for 0.0333333h; Green chemistry; | 98% |
| With succinimide-N-sulfonic acid In acetonitrile at 20℃; for 0.0333333h; chemoselective reaction; | 95% |
-
-
1666-13-3
diphenyl diselenide

-
-
1745-81-9
2-Allylphenol

-
-
66558-11-0
2,3-dihydro-2-<(phenylseleno)methyl>benzofuran

| Conditions | Yield |
|---|---|
| With (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile In acetonitrile at 20℃; for 2h; Irradiation; Green chemistry; | 98% |
| With ammonium persulfate In acetonitrile at 70℃; for 12h; | 77% |
| With tetrabutylammonium perchlorate In acetonitrile at 20℃; for 1.5h; Electrochemical reaction; Green chemistry; | 55% |
-
-
35787-71-4
thianthrene cation radical perchlorate

-
-
1745-81-9
2-Allylphenol

-
A
-
2362-50-7
thianthrene-5-oxide

-
B
-
139656-67-0
5-(4-hydroxy-3-allylphenyl)thianthreniumyl perchlorate

-
C
-
92-85-3
Thianthrene

| Conditions | Yield |
|---|---|
| In acetonitrile | A 5.4% B 98% C 40% |

-
-
1745-81-9
2-Allylphenol

-
-
24844-28-8, 59981-81-6, 111268-65-6, 123619-78-3, 123619-79-4, 21895-83-0
2-(prop-2-en-1-yl)cyclohexan-1-ol

| Conditions | Yield |
|---|---|
| With nickel(II) oxide; hydrogen; palladium In hexane at 100℃; under 22502.3 Torr; for 16h; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate at 0 - 20℃; for 0.166667h; Green chemistry; | 98% |
| With pyridine In dichloromethane | 52.6 mg |

| Conditions | Yield |
|---|---|
| With N,N'-dimethylpiperazine In 1,4-dioxane at 80℃; Inert atmosphere; Schlenk technique; | 98% |
| With 1,4-diaza-bicyclo[2.2.2]octane; fac-tris(2-phenylpyridinato-N,C2')iridium(III) In acetonitrile at 20℃; for 12h; Reagent/catalyst; Solvent; Schlenk technique; Irradiation; Inert atmosphere; | 90% |
| With 1,4-diaza-bicyclo[2.2.2]octane; tris[2-phenylpyridinato-C2,N]iridium(III) In acetonitrile at 25℃; for 12h; Schlenk technique; Irradiation; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0 - 20℃; for 0.333333h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; for 18h; | 98% |

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; potassium carbonate; triphenylphosphine In water at 85 - 105℃; for 16h; Inert atmosphere; | 98% |
| With 5%-palladium/activated carbon; potassium carbonate; triphenylphosphine In water at 85 - 105℃; for 4h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With benzo[de]benzo[4,5]imidazo[2,1-a]isoquinolin-7-one In cyclohexane at 20℃; for 1h; Inert atmosphere; Sealed tube; Irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With potassium methanolate at 110 - 123℃; for 18.3h; Inert atmosphere; | 97.1% |
2-Allylphenol Specification
1.Introduction of 2-Allylphenol
The 2-Allylphenol, with the CAS registry number 1745-81-9, is also known as o-Allylphenol. It belongs to the product categories of Benzene Derivatives; Organic Building Blocks; Oxygen Compounds; Phenols. Its EINECS registry number is 217-119-0. This chemical's molecular formula is C9H10O and molecular weight is 134.18. What's more, its IUPAC name is called 2-Prop-2-enylphenol. It should be stored in a cool, dry and well-ventilated place.
2.Physical properties about 2-Allylphenol
(1)ACD/LogP: 2.441; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.44; (4)ACD/LogD (pH 7.4): 2.44; (5)ACD/BCF (pH 5.5): 42.20; (6)ACD/BCF (pH 7.4): 42.15; (7)ACD/KOC (pH 5.5): 507.00; (8)ACD/KOC (pH 7.4): 506.37; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.548; (14)Molar Refractivity: 42.042 cm3; (15)Molar Volume: 132.281 cm3; (16)Polarizability: 16.667×10-24cm3; (17)Surface Tension: 37.650 dyne/cm; (18)Density: 1.014 g/cm3; (19)Flash Point: 88.889 °C; (20)Enthalpy of Vaporization: 47.5 kJ/mol; (21)Boiling Point: 219.999 °C at 760 mmHg; (22)Vapour Pressure: 0.078 mmHg at 25 °C.
3.Structure descriptors of 2-Allylphenol
(1) SMILES: Oc1ccccc1C/C=C
(2) InChI: InChI=1S/C9H10O/c1-2-5-8-6-3-4-7-9(8)10/h2-4,6-7,10H,1,5H2
(3) InChIKey: QIRNGVVZBINFMX-UHFFFAOYSA-N
4.Preparation of 2-Allylphenol
The 2-Allylphenol can be prepared by allyloxy-benzene. This reaction needs reagent 1,2-bis(3-chloro-2-hydroxy-5-methylbenzyl)benzene and solvent CH2Cl2 at temperature of 20 °C. The reaction is rearrangement reaction. The yield is 92 %.

5. Uses of 2-Allylphenol
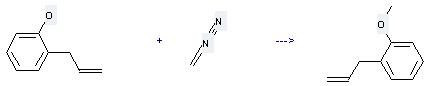
(1) it is used in organic synthesis; (2) it is used to produce other chemicals. For example, it can react with diazomethane to get 2-allyl-anisole. The reaction occurs with solvents diethyl ether and methanol at temperature of 0 °C. The yield is 45 %.
6.Safety information of 2-Allylphenol
This chemical may destroy living tissue on contact and may cause inflammation to the skin or other mucous membranes. It is harmful in contact with skin and if swallowed and it may cause burns. In addition, it is irritating to eyes and skin. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. You can not breathe the gas/fumes/vapour/spray and should avoid contacting with skin and eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell seek medical advice immediately.
Related Products
- 2-Allylphenol
- 1745-89-7
- 174590-39-7
- 174603-56-6
- 17460-56-9
- 174607-68-2
- 174609-74-6
- 1746-11-8
- 1746-13-0
- 1746-25-4
- 17462-58-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View