-
Name
2-Azetidinone
- EINECS
- CAS No. 930-21-2
- Article Data58
- CAS DataBase
- Density 1.119 g/cm3
- Solubility
- Melting Point 74-76 °C(lit.)
- Formula C3H5NO
- Boiling Point 222.434 °C at 760 mmHg
- Molecular Weight 71.0788
- Flash Point 151.536 °C
- Transport Information UN 3263 8/PG 2
- Appearance Colorless solid
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 2-Azetidone;Propiolactam;b-Alanine,lactam;b-Propiolactam;
- PSA 29.10000
- LogP -0.16490
Synthetic route
-
-
17392-83-5
(R)-Methyl lactate

-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| Stage #1: (R)-Methyl lactate With dmap; triethyl phosphite at 2 - 20℃; for 5.66667h; Stage #2: With sodium hydroxide at 55℃; for 1.5h; Temperature; | 95.6% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine In acetonitrile for 4.5h; Heating; | 80% |
| With sodium hydrogencarbonate; methanesulfonyl chloride In acetonitrile at 80℃; | 36% |
| With 2,2'-dipyridyldisulphide; triphenylphosphine In acetonitrile for 5h; Heating; | 0.44 g |
| In toluene at 90℃; Inert atmosphere; Green chemistry; |
-
-
130065-29-1
amino-azetidinone

-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| With N-nitrosodiphenylamine In benzene for 3h; Heating; | 61% |

| Conditions | Yield |
|---|---|
| With ruthenium(IV) oxide; sodium periodate In ethyl acetate for 24h; Ambient temperature; | A 35% B 42% |

| Conditions | Yield |
|---|---|
| With ruthenium(IV) oxide; sodium periodate In ethyl acetate for 72h; Ambient temperature; | 36% |

| Conditions | Yield |
|---|---|
| With triisobutylaluminum In diethyl ether at 45 - 50℃; for 15h; | 28% |
-
-
60-29-7
diethyl ether

-
-
924-73-2
3-amino-propionic acid ethyl ester

-
-
925-90-6
ethylmagnesium bromide

-
-
930-21-2
2-azetidinone


| Conditions | Yield |
|---|---|
| With diethyl ether anschliessend Behandeln mit wss.Ammoniumchlorid-Loesung; |
-
-
28562-53-0
4-acetoxy azetidinone

-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; acidic Amberlite resin 1.) ethanol, 0 deg C, 1 h; 2.) 0.5 h, 0 deg C; Yield given. Multistep reaction; | |
| With potassium borohydride In water | |
| With sodium borohydrid; sodium chloride In water; pentane | 8.7 g (61%) |
| In Potassium borohydride; dichloromethane; water |
-
-
115946-47-9
1-[(4-methylphenyl)sulfonyl]azetidin-2-one

-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| With acetic acid In N,N-dimethyl-formamide at 20℃; Hg-cathode, Et4NClO4; | 89 % Chromat. |

-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane; water at 20℃; |

-
A
-
930-21-2
2-azetidinone

-
B
-
344930-61-6
N-hydroxymethylazetidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium perchlorate In water at 25℃; pH=2; Kinetics; Further Variations:; Reagents; Temperatures; Solvents; pH-values; |

-
A
-
930-21-2
2-azetidinone

-
B
-
344930-61-6
N-hydroxymethylazetidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium perchlorate In water at 25℃; pH=2; Kinetics; Further Variations:; Reagents; pH-values; Solvents; |

-
A
-
930-21-2
2-azetidinone

-
B
-
344930-61-6
N-hydroxymethylazetidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium perchlorate In water at 25℃; pH=2; Kinetics; Further Variations:; Reagents; pH-values; Solvents; |

-
A
-
930-21-2
2-azetidinone

-
B
-
344930-61-6
N-hydroxymethylazetidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium perchlorate In water at 25℃; pH=2; Kinetics; Further Variations:; Reagents; pH-values; Solvents; |

-
A
-
930-21-2
2-azetidinone

-
B
-
344930-61-6
N-hydroxymethylazetidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium perchlorate In water at 25℃; pH=2; Kinetics; Further Variations:; Reagents; pH-values; Solvents; |

-
A
-
930-21-2
2-azetidinone

-
B
-
344930-61-6
N-hydroxymethylazetidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium perchlorate In water at 25℃; pH=2; Kinetics; Further Variations:; Reagents; pH-values; Solvents; |

-
A
-
930-21-2
2-azetidinone

-
B
-
344930-61-6
N-hydroxymethylazetidin-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium perchlorate In water at 25℃; pH=2; Kinetics; Further Variations:; Reagents; pH-values; Solvents; |

-
A
-
930-21-2
2-azetidinone

-
B
-
701910-95-4
ethyl 2-hydroxy-2-(2-oxo-azetidin-1-yl)acetate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium perchlorate In water at 25℃; pH=2; Kinetics; Further Variations:; Reagents; pH-values; Solvents; Temperatures; |

-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; |
-
-
873073-30-4
1-(4-chlorobenzoyl)-1-azetidin-2-one

-
A
-
930-21-2
2-azetidinone

-
B
-
4641-33-2
4-chlorobenzoate ion

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium chloride In water; acetonitrile at 30℃; Kinetics; |
-
-
873073-29-1
1-(4-methoxybenzoyl)-1-azetidin-2-one

-
A
-
930-21-2
2-azetidinone

-
B
-
16285-97-5
p-methoxybenzoate(1-)

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium chloride In water; acetonitrile at 30℃; Kinetics; |
-
-
873073-31-5
1-(4-nitrobenzoyl)-1-azetidin-2-one

-
A
-
930-21-2
2-azetidinone

-
B
-
2906-29-8
4-nitrobenzoate

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium chloride In water; acetonitrile at 30℃; Kinetics; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium chloride In water; acetonitrile at 30℃; Kinetics; Further Variations:; pH-values; Solvents; Temperatures; |

-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane |

-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 25 - 30℃; |
-
-
930-21-2
2-azetidinone

-
-
924-44-7
glyoxylic acid ethyl ester

-
-
701910-95-4
ethyl 2-hydroxy-2-(2-oxo-azetidin-1-yl)acetate

| Conditions | Yield |
|---|---|
| In toluene for 3h; Heating; | 100% |
-
-
930-21-2
2-azetidinone

-
-
868047-80-7
1-bromo-5-(tert-butyl)-2-methoxy-3-nitrobenzene

-
-
868047-81-8
1-(5-tert-butyl-2-methoxy-3-nitro-phenyl)-azetidin-2-one

| Conditions | Yield |
|---|---|
| With caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; tris-(dibenzylideneacetone)dipalladium(0) In 1,4-dioxane at 100℃; for 20h; | 100% |
-
-
930-21-2
2-azetidinone

-
-
98-03-3
thiophene-2-carbaldehyde

-
-
1394836-02-2
1-(thiophene-2-carbonyl)azatedin-2-one

| Conditions | Yield |
|---|---|
| With Shvo's Catalyst In toluene at 100℃; for 24h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With methyltrifluoromethyldioxirane In water at 0℃; for 0.5h; | 99% |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; acetic acid In acetonitrile at 60℃; for 3h; Electrochemical reaction; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic acid at 20℃; for 18h; | 98% |
-
-
930-21-2
2-azetidinone

-
-
57913-41-4
2-phenylethyl chloroformate

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran at -78 - 20℃; for 1.75h; | 98% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In 1,2-dichloro-ethane at 20℃; | 98% |

-
-
930-21-2
2-azetidinone

-
-
1309362-40-0
1-heptyl-3-iodoquinolin-4(1H)-one

-
-
1309362-56-8
1-heptyl-3-(2-oxoazetidin-1-yl)quinolin-4(1H)-one

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate; N,N`-dimethylethylenediamine In toluene at 135℃; for 1h; Ullmann type reaction; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 24h; Heating; | 96% |
| With triethylamine In dichloromethane at 20℃; | 66% |
| With triethylamine In dichloromethane at 35℃; for 0.166667h; Microwave irradiation; Inert atmosphere; |
-
-
930-21-2
2-azetidinone

-
-
766-85-8
3-methoxy-1-iodobenzene

-
-
61999-50-6
N-(3-methoxyphenyl)azetidin-2-one

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate In toluene at 18 - 140℃; for 20h; Goldberg-Buchwald cross-coupling; Inert atmosphere; Sealed; | 96% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate In toluene at 18 - 140℃; for 20h; Goldberg-Buchwald cross-coupling; Inert atmosphere; Sealed; | 96% |
| With D-glucose; (R,R)-N,N'-dimethyl-1,2-diaminocyclohexane; copper(ll) bromide; sodium t-butanolate In water at 50℃; Inert atmosphere; | 79% |

| Conditions | Yield |
|---|---|
| With Shvo's Catalyst In toluene at 100℃; for 24h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; for 16h; Arylation; | 95% |
| With tris(dibenzylideneacetone)dipalladium (0); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; for 16h; | 93% |
| With 1,1'-bis(diphenylphosphino)ferrocene; sodium t-butanolate; palladium diacetate In toluene at 120℃; for 48h; Phenylation; | 20% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; (S,S)-1,2-diaminocyclohexane In 1,4-dioxane at 110℃; for 23h; | 95% |
| With potassium phosphate; copper(l) iodide; (S,S)-1,2-diaminocyclohexane In 1,4-dioxane; dodecane at 110℃; for 23h; | 95% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate In toluene at 110℃; for 24h; | 95% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In 1,2-dichloro-ethane at 20℃; | 95% |

-
-
930-21-2
2-azetidinone

-
-
22445-41-6
3,5-dimethylphenyl iodide

| Conditions | Yield |
|---|---|
| 95% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate In toluene at 18 - 140℃; for 20h; Goldberg-Buchwald cross-coupling; Inert atmosphere; Sealed; | 95% |
-
-
930-21-2
2-azetidinone

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; trans-1,2-Diaminocyclohexane In 1,4-dioxane at 110℃; for 24h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With Ru-carbon; sodium acetate; acetic acid In ethyl acetate for 2.5h; Ambient temperature; | 94% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; for 18h; Arylation; | 94% |
-
-
930-21-2
2-azetidinone

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
117505-49-4
1-tert-butyldimethylsilylazetidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine at 0℃; for 1h; | 93% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 24h; Inert atmosphere; | 90% |
| With triethylamine In N,N-dimethyl-formamide at 0℃; for 0.5h; | 61% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-azetidinone With n-butyllithium; 1,1,1,3,3,3-hexamethyl-disilazane In tetrahydrofuran at -78℃; for 1.5h; Inert atmosphere; Stage #2: benzyl chloroformate In tetrahydrofuran at -78 - 20℃; for 6h; Inert atmosphere; | 93% |
| With lithium hexamethyldisilazane In tetrahydrofuran at -78 - 20℃; for 1.75h; | 75% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In 1,2-dichloro-ethane at 20℃; | 93% |

-
-
930-21-2
2-azetidinone

-
-
12080-32-9
dichloro(1,5-cyclooctadiene)platinum(ll)

-
-
188638-31-5
[Pt(C8H12)(C3H4NO)2]

| Conditions | Yield |
|---|---|
| With silver(I) oxide In dichloromethane reflux (4 h); filtration, concn., pptn. with light petroleum, filtration, washing (light petroleum), drying (vac.); elem. anal.; | 93% |
-
-
930-21-2
2-azetidinone

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1140510-99-1
tert-butyl 2-oxoazetidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With dmap In acetonitrile at 0 - 20℃; Inert atmosphere; | 93% |
| With dmap In acetonitrile at 0 - 20℃; Inert atmosphere; | 90% |
| With dmap; triethylamine In dichloromethane at 20℃; for 12h; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate In toluene at 18 - 140℃; for 20h; Goldberg-Buchwald cross-coupling; Inert atmosphere; Sealed; | 93% |

| Conditions | Yield |
|---|---|
| With Shvo's Catalyst In toluene at 100℃; for 24h; Inert atmosphere; | 93% |
2-Azetidinone Chemical Properties
Molecular Structure of 2-Azetidinone (CAS No.930-21-2):
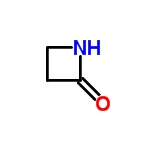
Molecular Formula: C3H5NO
Molecular Weight: 71.08
IUPAC Name: Azetidin-2-one
CAS No: 930-21-2
Melting Point: 74-76 ºC
H bond acceptors: 2
H bond donors: 1
Freely Rotating Bonds: 0
Polar Surface Area: 20.31 Å2
Index of Refraction: 1.457
Molar Refractivity: 17.31 cm3
Molar Volume: 63.5 cm3
Surface Tension: 34.4 dyne/cm
Density: 1.119 g/cm3
Flash Point: 151.5 °C
Enthalpy of Vaporization: 45.89 kJ/mol
Boiling Point: 222.4 °C at 760 mmHg
Vapour Pressure: 0.102 mmHg at 25°C
Storage Temp.: 2-8°C
Appearance: Colorless solid
Product Categories: Heterocycles series;Ring Systems
Canonical SMILES: C1CNC1=O
InChI: InChI=1S/C3H5NO/c5-3-1-2-4-3/h1-2H2,(H,4,5)
InChIKey: MNFORVFSTILPAW-UHFFFAOYSA-N
2-Azetidinone Safety Profile
Safety Information of 2-Azetidinone (CAS No.930-21-2):
Hazard Codes:  C
C
Risk Statements: 34
R34:Causes burns.
Safety Statements: 26-36/37/39-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 3263 8/PG 2
WGK Germany: 3
Hazard Note: Corrosive/Keep Cold
HazardClass: 8
PackingGroup: III
2-Azetidinone Specification
2-Azetidinone (CAS No.930-21-2), it also can be called 2-Azacyclobutanone ; 2-azetol, 3,4-dihydro- ; Azetidin-2-one . An invention relates to 2-azetidinone compounds in 1981, which have antimicrobial activities, and more particularly, this invention provides new 2-azetidinone compounds, especially ones having various substituted carboxyalkyl radicals at the first position and having various groups at the fourth position of the azetidinone nucleus, which have antimicrobial activities against various pathogenic microorganisms and are useful as antibiotics in treatment for microbial infections in mammal including human being and animals.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View