-
Name
2-Methyl-3-nitroaniline
- EINECS 210-059-6
- CAS No. 603-83-8
- Article Data29
- CAS DataBase
- Density 1.269 g/cm3
- Solubility <0.1 g/100 mL at 23 °C in water
- Melting Point 88-90 °C(lit.)
- Formula C7H8N2O2
- Boiling Point 304.7 °C at 760 mmHg
- Molecular Weight 152.153
- Flash Point 138.1 °C
- Transport Information UN 2660 6.1/PG 3
- Appearance Yellow to greenish-yellow crystalline powder
- Safety 28-36/37-45-61-28A
- Risk Codes 23/24/25-33-51/53
-
Molecular Structure
-
Hazard Symbols
 T,
T,  N
N
- Synonyms o-Toluidine,3-nitro- (8CI);1-Amino-2-methyl-3-nitrobenzene;2-Amino-6-nitrotoluene;2-Methyl-3-nitrobenzenamine;3-Nitro-o-toluidine;6-Amino-2-nitrotoluene;6-Nitro-2-aminotoluene;NSC 227939;NSC 25011;
- PSA 71.84000
- LogP 2.58980
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dinitrotoluene In 1,4-dioxane; water at 50℃; for 0.0833333h; Stage #2: With formic acid In 1,4-dioxane; water at 50℃; for 2h; Sealed tube; | 95% |
| With ammonium sulfide In ethanol at 79℃; for 2h; Reflux; | 79% |
| With formic acid; triethylamine; palladium on activated charcoal at 100℃; for 0.333333h; | 76% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid |
-
-
2464-33-7
N-(2-methylphenyl)phthalimide

-
-
7664-41-7
ammonia

-
A
-
603-83-8
3-nitro-o-tolylamine

-
B
-
99-55-8
2-methyl-5-nitroaniline

| Conditions | Yield |
|---|---|
| at 100℃; Zuerst nitrieren die Ausgangverbindung; |


| Conditions | Yield |
|---|---|
| Nitrieren und Verseifung der entstandenen Phthalylnitrotoluidine mit alkoh.Ammoniak im Autoklaven bei 100grad; | |
| durch Nitrieren und Verseifen der gebildeten Phthalylnitrotoluidine mit alkoh.Ammoniak bei 100grad; |
-
-
7664-93-9
sulfuric acid

-
-
95-53-4
o-toluidine

-
A
-
603-83-8
3-nitro-o-tolylamine

-
B
-
99-52-5
2-methyl-4-nitro-benzenamine

-
C
-
99-55-8
2-methyl-5-nitroaniline

| Conditions | Yield |
|---|---|
| beim Nitrieren; |


| Conditions | Yield |
|---|---|
| bei der elektrolytischen Reduktion; |


| Conditions | Yield |
|---|---|
| Hydrogenation; |


-
-
64-17-5
ethanol

-
-
606-20-2
2,6-dinitrotoluene

-
A
-
5805-95-8
2-hydroxylamino-6-nitrotoluene

-
B
-
603-83-8
3-nitro-o-tolylamine

| Conditions | Yield |
|---|---|
| Hydrogenation; |

-
-
606-20-2
2,6-dinitrotoluene

-
-
7783-06-4
hydrogen sulfide

-
-
7664-41-7
ammonia

-
A
-
603-83-8
3-nitro-o-tolylamine

| Conditions | Yield |
|---|---|
| Behandlung des Reaktionsproduktes mit Salzsaeure; |

-
-
541-41-3
chloroformic acid ethyl ester

-
-
603-83-8
3-nitro-o-tolylamine

-
-
381670-28-6
(2-methyl-3-nitro-phenyl)-carbamic acid ethyl ester

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 65℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium nitrite In water; acetic acid | 99% |
| With acetic acid; sodium nitrite at 20℃; | 95% |
| With sodium nitrite In water; acetic acid at 45 - 55℃; for 1h; Large scale; | 95% |

| Conditions | Yield |
|---|---|
| at 100℃; for 3h; | 99% |
-
-
50329-91-4
4-chloro-3-formylcoumarin

-
-
603-83-8
3-nitro-o-tolylamine

-
-
1394232-20-2
4-(2-hydroxyphenyl)-8-methyl-7-nitroquinoline-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol at 20℃; for 0.0833333h; | 99% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
1061566-50-4
4,6-dideuterio-2-methyl-3-nitroaniline

| Conditions | Yield |
|---|---|
| Stage #1: 3-nitro-o-tolylamine With hydrogenchloride; water-d2 at 180℃; for 0.5h; Microwave irradiation; Stage #2: With sodium hydroxide In diethyl ether; water regioselective reaction; | 97% |
-
-
89-98-5
2-chloro-benzaldehyde

-
-
603-83-8
3-nitro-o-tolylamine

-
-
128422-36-6
2-(2-chlorobenzylideneamino)-6-nitrotoluene

| Conditions | Yield |
|---|---|
| In ethanol for 5h; Heating; | 96% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
117824-52-9
4,6-dibromo-2-methyl-3-nitro-aniline

| Conditions | Yield |
|---|---|
| With benzyltrimethylammonium tribromide; calcium carbonate In methanol; dichloromethane for 0.5h; Ambient temperature; | 95% |
| With bromine In acetic acid for 1h; heated on a steam bath; | 84% |
| With bromine; acetic acid |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
122-51-0
orthoformic acid triethyl ester

-
-
115118-93-9
N-(2-methyl-3-nitrophenyl)ethoxymethylenimine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 120℃; for 1h; | 94% |
| With sodium hydrogen tartrate at 102℃; for 2h; Time; | 90.84% |
| With toluene-4-sulfonic acid at 120℃; for 1h; Claisen Condensation; | 88% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
41252-98-6
2-iodo-6-nitrotoluene

| Conditions | Yield |
|---|---|
| diazotization; | 93% |
| Stage #1: 3-nitro-o-tolylamine With sulfuric acid; sodium nitrite at 0 - 20℃; for 2h; Stage #2: With potassium iodide at 20℃; for 12h; Further stages.; | 91% |
| Stage #1: 3-nitro-o-tolylamine With sulfuric acid; sodium nitrite In water; acetonitrile at -7 - -5℃; Inert atmosphere; Stage #2: With potassium iodide In water; acetonitrile at 0 - 20℃; for 0.333333h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With acetic acid for 5h; Condensation; Heating; | 92.5% |
| In pyridine |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
78-39-7
Triethyl orthoacetate

-
-
133053-71-1
N-(2-methyl-3-nitro-phenyl)-acetimidic acid ethyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 120℃; | 92% |
| With toluene-4-sulfonic acid at 120℃; for 1h; | 73% |
| With toluene-4-sulfonic acid at 120℃; for 1h; |
-
-
108-31-6
maleic anhydride

-
-
603-83-8
3-nitro-o-tolylamine

-
-
221279-43-2
N-(2-methyl-3-nitrophenyl)maleamic acid

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; | 92% |
| In diethyl ether for 60h; | 91% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
55289-35-5
2-bromo-6-nitrotoluene

| Conditions | Yield |
|---|---|
| Stage #1: 3-nitro-o-tolylamine With hydrogen bromide for 0.333333h; Heating; Stage #2: With sodium nitrite at 0 - 5℃; for 0.25h; Stage #3: With hydrogen bromide; copper(I) bromide at 100℃; for 0.333333h; | 90% |
| Stage #1: 3-nitro-o-tolylamine With hydrogen bromide In water for 0.166667h; Reflux; Stage #2: With sodium nitrite In water at 0 - 5℃; for 0.5h; Stage #3: With copper(I) bromide In water at 20 - 70℃; for 1.5h; | 89% |
| Stage #1: 3-nitro-o-tolylamine With hydrogen bromide; sodium nitrite In water at 0 - 5℃; for 0.333333 - 0.5h; Sandmeyer Reaction; Reflux; Stage #2: With hydrogen bromide; copper(I) bromide In water at 30 - 35℃; for 0.5h; Sandmeyer Reaction; Reflux; | 84% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
124-63-0
methanesulfonyl chloride

-
-
80259-07-0
N-(2-methyl-3-nitrophenyl)methanesulfonamide

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 16h; | 90% |
| With pyridine at 20℃; for 2h; | |
| In pyridine |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
98-88-4
benzoyl chloride

-
-
133053-84-6
N-(2-methyl-3-nitrophenyl)-benzamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; | 89% |
| With pyridine for 0.5h; Ambient temperature; | 78% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
122-51-0
orthoformic acid triethyl ester

-
-
115118-93-9
ethyl N-(2-methyl-3-nitrophenyl)formimidate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid | 89% |
| With toluene-4-sulfonic acid Yield given; |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
115-80-0
Triethyl orthopropionate

-
-
87885-86-7
ethyl N-(2-methyl-3-nitrophenyl)proponimidate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 120℃; for 1h; | 89% |
-
-
108-24-7
acetic anhydride

-
-
603-83-8
3-nitro-o-tolylamine

-
-
86009-37-2
1-(4-nitro-1H-indazol-1-yl)ethan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-nitro-o-tolylamine With potassium acetate In chloroform at 20℃; Stage #2: acetic anhydride In chloroform at 0 - 40℃; Stage #3: With isopentyl nitrite In chloroform Reflux; | 89% |
| Stage #1: acetic anhydride; 3-nitro-o-tolylamine With potassium acetate In toluene at 25℃; for 0.25h; Inert atmosphere; Stage #2: With isopentyl nitrite In toluene at 25℃; Inert atmosphere; Reflux; | 60% |
| Stage #1: acetic anhydride; 3-nitro-o-tolylamine With potassium acetate In toluene at 20℃; Stage #2: With isopentyl nitrite Reflux; |

| Conditions | Yield |
|---|---|
| Stage #1: 3-nitro-o-tolylamine With hydrogenchloride; sodium nitrite In water; acetonitrile at -10 - -5℃; for 0.5h; Stage #2: 2-acetylpropanoic acid ethyl ester With potassium hydroxide In ethanol; water; acetonitrile at -6 - 2℃; for 0.166667h; Stage #3: With hydrogenchloride In ethanol; water; acetonitrile at 0℃; for 1h; | 88% |
| With hydrogenchloride; sodium nitrite Multistep reaction; |
-
-
862374-44-5
4-(3-(allyloxy)-4-fluorophenoxy)benzaldehyde

-
-
603-83-8
3-nitro-o-tolylamine

-
-
448957-16-2
N-(4-(3-(allyloxy)-4-fluorophenoxy)benzyl)-N-(2-methyl-3-nitrophenyl)amine

| Conditions | Yield |
|---|---|
| Stage #1: 4-(3-(allyloxy)-4-fluorophenoxy)benzaldehyde; 3-nitro-o-tolylamine With acetic acid In dichloromethane at 20℃; for 4h; Stage #2: With sodium tris(acetoxy)borohydride In dichloromethane at 20℃; for 12h; | 88% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
56682-03-2
1,2-bis(2-methyl-3-nitrophenyl)disulfane

| Conditions | Yield |
|---|---|
| Stage #1: 3-nitro-o-tolylamine With tetrafluoroboric acid; sodium nitrite In water; acetone at -20 - 0℃; for 0.333333h; Stage #2: With carbon disulfide; tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate In dimethyl sulfoxide at 20℃; for 6h; Irradiation; | 88% |
| Multi-step reaction with 2 steps 1: (i) (diazotization), (ii) /BRN= 3561118/, KSCN 2: NH3 / ethanol View Scheme |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
-
52979-48-3
dimethyl 2-(2-methyl-3-nitrophenylamino)-2-butenedioate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 2h; | 88% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
414868-82-9
4-bromo-2-methyl-3-nitroaniline

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In acetonitrile at 0℃; for 0.5h; | 88% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
375856-45-4
N-benzyl-2-methyl-3-nitroaniline

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; benzaldehyde In 1,1-dichloroethane; acetic acid | 87% |
| Multi-step reaction with 2 steps 1: AcOH / 1,2-dichloro-ethane / 20 °C 2: Na(OAc)3BH / 1,2-dichloro-ethane View Scheme | |
| Multi-step reaction with 2 steps 1: AcOH / 1,2-dichloro-ethane / 4 h / 20 °C 2: Na(OAc)3BH / 1,2-dichloro-ethane / 12 h View Scheme |
-
-
50-00-0
formaldehyd

-
-
603-83-8
3-nitro-o-tolylamine

-
-
1142382-04-4
(rac)-4,10-dimethyl-3,9-dinitro-5,11-methano-6H,12H-dibenzo[b,f][1,5]diazocine

| Conditions | Yield |
|---|---|
| In trifluoroacetic acid at 20℃; for 48h; | 87% |
| With trifluoroacetic acid for 48h; | 87% |
| With trifluoroacetic acid at 20℃; for 48h; | 85% |
-
-
603-83-8
3-nitro-o-tolylamine

-
-
4635-59-0
4-Chlorobutanoyl chloride

-
-
133053-93-7
4-chloro-N-(2-methyl-3-nitrophenyl)-butyramide

| Conditions | Yield |
|---|---|
| With pyridine In diethyl ether for 3h; | 86% |
-
-
100-39-0
benzyl bromide

-
-
603-83-8
3-nitro-o-tolylamine

-
-
448956-36-3
N,N-dibenzyl-N-(2-methyl-3-nitrophenyl)amine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide | 86% |
-
-
50-00-0
formaldehyd

-
-
1635-31-0
aminocoumarine

-
-
603-83-8
3-nitro-o-tolylamine

-
-
762-42-5
dimethyl acetylenedicarboxylate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 3h; Reflux; | 86% |


| Conditions | Yield |
|---|---|
| With melamine-formaldehyde resin supported H+ In neat (no solvent) at 80℃; for 0.133333h; | 84% |
2-Methyl-3-nitroaniline Chemical Properties
IUPAC Name: 2-Methyl-3-nitroaniline
Product Name: 2-Methyl-3-nitroaniline
The MF of 2-Methyl-3-nitroaniline (CAS NO.603-83-8) is C7H8N2O2.
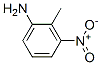
The MW of 2-Methyl-3-nitroaniline (CAS NO.603-83-8) is 152.15.
Synonyms of 2-Methyl-3-nitroaniline (CAS NO.603-83-8): 2-Methyl-3-aminonitrobenzene ; 2-Methyl-3-nitroaniline ; 3-Nitro-o-toluidine ; Benzenamine, 2-methyl-3-nitro- (9CI) ; o-Toluidine, 3-nitro- ; 2-Amino-6-nitrotoluene
Product Categories: Intermediates of Dyes and Pigments;Benzene derivatives;Anilines, Aromatic Amines and Nitro Compounds;API intermediates;Drug Intermediates
Form: Yellow rhombic needles (from water) or bright yellow powder
Water Solubility: <0.1 g/100 mL at 23 ºC
Index of Refraction: 1.615
EINECS: 210-059-6
Density: 1.269 g/ml
Flash Point: 138.1 °C
Boiling Point: 304.7 °C
Melting Point: 88-91 °C
BRN: 388393
2-Methyl-3-nitroaniline Uses
2-Methyl-3-nitroaniline (CAS NO.603-83-8) is mainly used for cotton dyeing and printing of color reagent.
2-Methyl-3-nitroaniline Safety Profile
Safety information of 2-Methyl-3-nitroaniline (CAS NO.603-83-8):
Hazard Codes  T,
T, N
N
Risk Statements
23/24/25 Toxic by inhalation, in contact with skin and if swallowed
33 Danger of cumulative effects
51/53 Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
Safety Statements
28 After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer)
36/37 Wear suitable protective clothing and gloves
45 In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
61 Avoid release to the environment. Refer to special instructions safety data sheet
RIDADR UN 2660 6.1/PG 3
WGK Germany 3
RTECS XU8200000
Hazard Note Toxic
HazardClass 6.1
PackingGroup III
HS Code 29214300
2-Methyl-3-nitroaniline Specification
2-Methyl-3-nitroaniline is incompatible with acids, acid chlorides, acid anhydrides, chloroformates and strong oxidizers.
Related Products
- 2-Methyl-3-nitroaniline
- 60384-53-4
- 603-84-9
- 603-85-0
- 60385-84-4
- 6038-59-1
- 603-86-1
- 60-38-8
- 60388-02-5
- 60388-36-5
- 60388-38-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View