-
Name
2-Nitrophenol
- EINECS 201-857-5
- CAS No. 88-75-5
- Article Data527
- CAS DataBase
- Density 1.395 g/cm3
- Solubility 2 g/L (25 °C) in water
- Melting Point 43-47 °C
- Formula C6H5NO3
- Boiling Point 215.8 °C at 760 mmHg
- Molecular Weight 139.111
- Flash Point 97.1 °C
- Transport Information UN 1663 6.1/PG 3
- Appearance yellow crystalline solid
- Safety 26-61-45-36/37-16-7-36-28
- Risk Codes 22-36/37/38-52/53-33-20/21/22-39/23/24/25-23/24/25-11
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  T,
T,  F
F
- Synonyms Phenol,o-nitro- (8CI);2-Hydroxynitrobenzene;NSC 1552;o-Hydroxynitrobenzene;o-Nitrophenol;CCRIS 2314;CHEBI:16260;NSC 1552;Ortho-nitrophenol;
- PSA 66.05000
- LogP 1.82360
Synthetic route

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 22h; Heating; | A 100% B 98% |
-
-
25458-47-3
methoxy-(2-nitro-phenoxy)-methane

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| sodium hydrogen sulfate; silica gel In dichloromethane at 20℃; for 1h; | 100% |
| With Montmorillonite K 10 In benzene at 25℃; for 5h; | 93% |

| Conditions | Yield |
|---|---|
| With pentamethylbenzene,; boron trichloride In dichloromethane at -78℃; for 0.333333h; | 100% |
| With methyl-phenyl-thioether; trifluoroacetic acid In toluene at 20℃; for 3h; | 97% |
| With thiophene; sodium hydrogen sulfate; silica gel for 4.5h; Heating; | 94% |
| With palladium diacetate; sodium hydride In N,N-dimethyl acetamide at 25℃; for 3h; Inert atmosphere; | 78% |
| With sulfosuccinic acid functionalized mesoporous silica In ethanol at 100℃; |

| Conditions | Yield |
|---|---|
| With [CuII2(μ-OH)(1,2-bis(2-(bis(2-pyridylmethyl)aminomethyl)-6-pyridyl)ethane)](ClO4)3 ; dihydrogen peroxide; triethylamine In water; acetonitrile at 50℃; for 40h; Catalytic behavior; Inert atmosphere; | 99% |
| bei der Einw. von Radiumstrahlen; | |
| With H5PV2Mo10O40(1,11); oxygen at 140℃; under 1500.12 Torr; Kinetics; Further Variations:; Pressures; reagent concentration; |

| Conditions | Yield |
|---|---|
| With 1-methyl-2-pyridone; dinitrogen tetroxide; Nitrogen dioxide In acetonitrile | 98% |
| With nitric acid In 1,2-dichloro-ethane at 25℃; for 3h; Catalytic behavior; Kinetics; Reagent/catalyst; Solvent; Temperature; Time; regioselective reaction; | 98.3% |
| With ammonium cerium(IV) nitrate for 0.0333333h; microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 6h; Heating; | 98% |
| With water; hydrogen bromide; Aliquat 336 at 105℃; for 4.5h; Catalytic behavior; | 92% |
| With copper(I) oxide; sodium methylate In methanol at 185℃; for 12h; Autoclave; | 87% |
| With pyridine hydrochloride for 0.266667h; demethylation; microwave irradiation; | 65% |
-
-
158813-51-5
tert-butyldimethyl(2-nitrophenoxy)silane

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With lithium acetate In water; N,N-dimethyl-formamide at 25℃; for 1.5h; Inert atmosphere; | 98% |
| sulfated SnO2 In methanol at 20℃; for 0.166667h; | 96% |
| With potassium-exchanged zirconium hydrogen phosphate In water; acetone at 60℃; for 3.5h; desilylation; | 76% |

| Conditions | Yield |
|---|---|
| With copper In 1-methyl-pyrrolidin-2-one at 250℃; for 0.5h; | 98% |
-
-
91493-71-9
benzenesulfonic acid-(2-nitro-phenyl ester)

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In toluene; tert-butyl alcohol at 100℃; for 0.5h; Inert atmosphere; | 97% |
-
-
55339-51-0
2-allyloxy-1-nitrobenzene

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 20℃; for 8h; | 96% |
| With palladium diacetate; sodium hydride In N,N-dimethyl acetamide; mineral oil at 10℃; for 8h; Inert atmosphere; | 92% |
| With palladium diacetate; sodium hydride In N,N-dimethyl acetamide at 20℃; for 4h; Inert atmosphere; | 72% |

| Conditions | Yield |
|---|---|
| With silica supported Al(NO3)3*9H2O In acetone at 20℃; for 0.25h; Reagent/catalyst; Solvent; regioselective reaction; | A 3% B 95% |
| With nitro acetate; silica gel In chloroform at -20 - 20℃; Nitration; | A 7% B 93% |
| With nitrate of N-dodecyl-pyridinium 3-carboxylate In acetonitrile Product distribution; Mechanism; investigation of nitration of several phenols with NO2-N2O4 in presence of pyridine derivatives in var. solvents; | A 5% B 92% |

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate In water at 20℃; for 2h; Schlenk technique; | 95% |

| Conditions | Yield |
|---|---|
| With 2-(methylsulfonyl)ethyl alcohol; sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; | 94% |
| With sodium trimethylsilanolate In tetrahydrofuran Substitution; addition under a nitrogen atmosphere, then reflux for 36 h; | 75% |
| With methyl propargyl alcohol; potassium tert-butylate In dimethyl sulfoxide at 125℃; for 0.0333333h; microwave irradiation; | 72% |

| Conditions | Yield |
|---|---|
| With iron(III) oxide; oxygen In tetrahydrofuran Irradiation; | 94% |
| With iron(III) oxide; oxygen In tetrahydrofuran at 20℃; Irradiation; | 94% |
| With C18H28Cl2CuN2O4 In water at 26℃; for 0.333333h; | 93% |
-
-
1198597-44-2
2-O2NC6H4OTIPS

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With potassium acetate In water; N,N-dimethyl-formamide at 25℃; for 1h; | 92% |

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| Stage #1: 2-<(nitrophenyl)oxy>pyridine With methyl trifluoromethanesulfonate In toluene at 100℃; for 2h; Inert atmosphere; Stage #2: With methanol; sodium for 0.25h; Concentration; Reflux; Inert atmosphere; | 92% |
| Stage #1: 2-<(nitrophenyl)oxy>pyridine With methyl trifluoromethanesulfonate In toluene at 90℃; for 2h; Inert atmosphere; Stage #2: With sodium In methanol; toluene at 80℃; for 0.5h; Temperature; Inert atmosphere; | 88% |
| Stage #1: 2-<(nitrophenyl)oxy>pyridine With methyl trifluoromethanesulfonate In toluene at 90℃; for 3h; Inert atmosphere; Stage #2: With sodium In methanol at 70℃; for 1h; Temperature; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tri-tert-butyl phosphine; tris-(dibenzylideneacetone)dipalladium(0) In toluene at 20 - 110℃; for 20h; | 91% |
| With potassium hydroxide In tert-butyl alcohol for 10h; Heating; | 70% |
| Stage #1: 2-Chloronitrobenzene With copper(ll) sulfate pentahydrate; water; sodium L-ascorbate; potassium hydroxide In dimethyl sulfoxide at 120℃; for 24h; Schlenk technique; Green chemistry; Stage #2: With hydrogenchloride In water; dimethyl sulfoxide at 20℃; for 0.5h; pH=2 - 3; Catalytic behavior; Schlenk technique; Green chemistry; | 63% |
| With sodium hydroxide; sodium carbonate at 130℃; | |
| With sodium hydroxide at 140℃; |

| Conditions | Yield |
|---|---|
| With sulfolane; sodium hydrogencarbonate at 200℃; for 2h; | 91% |

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 22h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With oxygen; nitric acid; 2,3-dicyano-5,6-dichloro-p-benzoquinone In water; acetonitrile at 30℃; for 24h; Reagent/catalyst; Temperature; Irradiation; Large scale; | A n/a B n/a C 90% |


| Conditions | Yield |
|---|---|
| With 1,9-diperoxynonanedioic acid In acetonitrile at 50℃; for 0.5h; | 89% |
| With caro's acid; diethyl ether | |
| With sodium peroxide; water | |
| With AcONa buffer; chloroperoxidase (EC 1.11.1.10) from Serratia marcescens; dihydrogen peroxide In water at 30℃; for 20h; | 5 % Chromat. |
| With 1-butyl-3-methylimidazolium phosphotungstate; 3-chloro-benzenecarboperoxoic acid In acetonitrile at 85℃; for 2h; |
-
-
98-54-4
para-tert-butylphenol

-
-
132993-22-7
2-nitrophenyl trifluoromethanesulfonate

-
A
-
154318-75-9
4-tert-butylphenyl triflate

-
B
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 10h; Ambient temperature; | A 89% B n/a |


| Conditions | Yield |
|---|---|
| With copper(I) oxide; 1-D-O-Methyl-chiro-inositol; sodium hydroxide In ethanol; water at 100℃; for 12h; | 88% |
| With copper(l) iodide; lithium pipecolinate; tetrabutyl ammonium fluoride; sodium hydroxide In water at 130℃; for 24h; | 85% |
| With lithium salt of proline; tetrabutylammomium bromide; potassium hydroxide; copper dichloride In water at 120℃; for 0.666667h; Microwave irradiation; Green chemistry; | 84% |
-
-
89466-08-0
2-hydroxyphenyl boronic acid

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With Iron(III) nitrate nonahydrate In toluene at 80℃; for 18h; Inert atmosphere; | 88% |
| With 1,1,1,3',3',3'-hexafluoro-propanol; N–nitrosaccharin at 60℃; for 19h; Inert atmosphere; Schlenk technique; | 70% |
-
-
16947-69-6
2,4,5-Trichlorophenyl chloroformate

-
-
460-08-2
2-fluoroethylamine hydrochloride

-
A
-
81420-42-0
mononitrophenyl carbonate

-
C
-
91390-34-0
2,4,5-trichlorophenyl (2-fluoroethyl)carbamate

-
D
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In diethyl ether for 0.75h; | A n/a B n/a C 85% D n/a |


| Conditions | Yield |
|---|---|
| Stage #1: 2-nitrophenyl bromide With copper(l) iodide; tetra(n-butyl)ammonium hydroxide In water at 60℃; for 24h; Inert atmosphere; Sealed tube; Stage #2: With hydrogenchloride In water; ethyl acetate at 20℃; for 2h; Inert atmosphere; chemoselective reaction; | 84% |
| With potassium phosphate; tri-tert-butyl phosphine; tris-(dibenzylideneacetone)dipalladium(0) In toluene at 20℃; for 20h; | 83% |
| With potassium hydroxide im Druckrohr; |
-
-
100-66-3
methoxybenzene

-
A
-
100-17-4
para-methoxynitrobenzene

-
B
-
91-23-6
2-Nitroanisole

-
C
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With dinitrogen tetraoxide In dichloromethane at -30℃; for 1h; Nitration; demethylation; | A n/a B 81% C 7% |


| Conditions | Yield |
|---|---|
| silica gel; toluene-4-sulfonic acid In water; toluene at 80℃; for 22h; | 80% |
| With lithium aluminium tetrahydride In diethyl ether for 2h; Heating; | 70% |
| With potassium chloride; poly(ethyleneimine) In water at 25℃; Rate constant; Mechanism; Product distribution; various pH, aminolysis; | |
| With Cu(N,N,N'-trimethyl-N'-tetradecylethylenediamine)Cl2 In acetonitrile at 31℃; Rate constant; also in pure water, var. concentration of reagent, pH 7.0; |
-
-
17258-49-0
1-(3'-methylbut-2'-enyloxy)-2-nitrobenzene

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With cerium(III) chloride; sodium iodide In acetonitrile for 3h; dealkylation; Heating; | 80% |
| With ytterbium(III) triflate In nitromethane for 12h; Ambient temperature; | 72% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 100% |
| With potassium carbonate In acetonitrile at 70℃; for 24h; Schlenk technique; | 99% |
| With potassium carbonate In acetone at 56 - 57℃; for 4h; | 99.5% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; C18H16Br4N2O3V; dihydrogen peroxide; potassium bromide In methanol; water at 20℃; for 0.333333h; Catalytic behavior; Reagent/catalyst; | 100% |
| With sulfuric acid; C20H22Br2N2O5V; dihydrogen peroxide In methanol; water at 20℃; for 0.583333h; Catalytic behavior; Reagent/catalyst; Solvent; | 100% |
| With N-bromosaccharin In tetrahydrofuran at 0℃; for 0.166667h; regioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen; Pd in AV-17-8-Pd In ethanol at 40℃; | 100% |
| With hydrogen; Pd in AV-17-8-Pd In ethanol at 40℃; under 760 Torr; Rate constant; | 100% |
| With hydrogen; palladium-containing anion exchanger AB-17-8-Pd In ethanol at 20 - 40℃; under 750.06 Torr; Kinetics; Product distribution; Further Variations:; Catalysts; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxynitrobenzene With potassium hydroxide In ethanol for 1h; Stage #2: ethyl bromoacetate In N,N-dimethyl-formamide for 24h; | 100% |
| Stage #1: 2-hydroxynitrobenzene With potassium hydroxide In methanol Stage #2: ethyl bromoacetate In methanol; N,N-dimethyl-formamide | 99% |
| Stage #1: 2-hydroxynitrobenzene With potassium hydroxide In ethanol for 1h; Stage #2: ethyl bromoacetate In N,N-dimethyl-formamide for 24h; Further stages.; | 99% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; tetra-(n-butyl)ammonium iodide; potassium carbonate In acetonitrile at 20 - 80℃; Inert atmosphere; | 100% |
| With 18-crown-6 ether; tetra-(n-butyl)ammonium iodide; potassium carbonate In acetonitrile at 20 - 80℃; Inert atmosphere; | 100% |
| With sodium hydride In N,N-dimethyl-formamide 1.) 0 deg C, 1 h, 2.) reflux, 24 h; | 60% |
| With potassium carbonate In acetone |
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
88-75-5
2-hydroxynitrobenzene

-
-
132993-22-7
2-nitrophenyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 0.5h; | 100% |
| With pyridine In dichloromethane at 0 - 25℃; Inert atmosphere; | 97% |
| With triethylamine In dichloromethane at 0 - 25℃; for 5h; Inert atmosphere; | 92% |
-
-
56512-49-3
dabsyl chloride

-
-
88-75-5
2-hydroxynitrobenzene

-
-
146303-70-0
4-(4-Dimethylamino-phenylazo)-benzenesulfonic acid 2-nitro-phenyl ester

| Conditions | Yield |
|---|---|
| With carbonate-bicarbonate buffer In acetone; acetonitrile 1.) 15 min, 2.) reflux; | 100% |
| With carbonate-bicarbonate buffer In acetone for 0.5h; Heating; |
-
-
106-96-7
propargyl bromide

-
-
88-75-5
2-hydroxynitrobenzene

-
-
13350-09-9
1-nitro-2-(prop-2-yn-1-yloxy)benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran | 100% |
| With caesium carbonate In acetonitrile at 20℃; for 24h; | 100% |
| In N,N-dimethyl-formamide at 20℃; for 18h; | 99% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
88-75-5
2-hydroxynitrobenzene

-
-
158813-51-5
tert-butyldimethyl(2-nitrophenoxy)silane

| Conditions | Yield |
|---|---|
| With pyridine for 24h; | 100% |
| With pyridine | 99% |
| With pyridine at 20℃; Inert atmosphere; | 94% |
| With pyridine at 20℃; Inert atmosphere; | 94% |
| With sodium hydroxide; Amberlite IRA-400 (chloride form) 2.) CH2Cl2, from 0 deg C to RT, 36 h; Yield given. Multistep reaction; |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
123-38-6
propionaldehyde

-
-
104-86-9
4-chlorobenzylamine

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| In methanol microwave irradiation; | 100% |
| In toluene at 60℃; for 20h; Ugi-Smiles coupling; | 90% |
| In methanol at 40℃; for 20h; Ugi reaction; | 74% |


| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 20℃; for 1h; | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
88-75-5
2-hydroxynitrobenzene

-
-
1040380-21-9
1-(tert-butoxycarbonyloxy)-2-nitrobenzene

| Conditions | Yield |
|---|---|
| With 6,7-dimethoxyisoquinoline In dichloromethane at 20℃; for 5h; Inert atmosphere; chemoselective reaction; | 100% |
| triphenylphosphine at 65℃; for 2h; | 98% |
| With carbon tetrabromide at 65℃; for 12h; Neat (no solvent); chemoselective reaction; | 88% |
| With MgO-ZrO2 nanoparticle at 60℃; for 2h; neat (no solvent); chemoselective reaction; | 82% |
-
-
181289-33-8
(3R)‐3‐(carbamoylmethyl)‐5‐methylhexanoic acid

-
-
88-75-5
2-hydroxynitrobenzene

-
-
1237492-12-4
C15H20N2O5

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 12h; Inert atmosphere; | 100% |
-
-
501-53-1
benzyl chloroformate

-
-
88-75-5
2-hydroxynitrobenzene

-
-
6132-44-1
carbonic acid benzyl ester-(2-nitro-phenyl ester)

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In ethyl acetate at 0 - 20℃; for 1.25h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: (R)-Methyl lactate; 2-hydroxynitrobenzene With triphenylphosphine In dichloromethane at 0℃; for 0.166667h; Mitsunobu Displacement; Stage #2: With diethylazodicarboxylate In dichloromethane at 0 - 20℃; for 8h; Mitsunobu Displacement; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: (S)-Methyl lactate; 2-hydroxynitrobenzene With triphenylphosphine In dichloromethane at 0℃; for 0.166667h; Mitsunobu Displacement; Stage #2: With diethylazodicarboxylate In dichloromethane at 0 - 20℃; for 8h; Mitsunobu Displacement; | 100% |

| Conditions | Yield |
|---|---|
| With 4-(N,N-dimethylamino)pyridine based 1,3,5,7-tetraphenyladamantane polymer In neat (no solvent) at 25℃; for 2.5h; Schlenk technique; | 99% |
| With 4-(dimethylamino)pyridine hydrochloride In toluene at 60℃; for 4h; | 98% |
| With carbon-silica composite from starch (7.5 molpercent SO3H) at 60℃; for 4h; solvent-free conditions; | 96% |

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In dichloromethane at 0 - 20℃; for 0.333333h; | 99% |
| With triphenylphosphine In acetonitrile for 5h; Heating; | 81% |
| With 1H-imidazole; iodine; chloro-diphenylphosphine In acetonitrile for 5h; Reflux; | 75% |
| Reaktion ueber mehrere Stufen; | |
| With 5,5'-dimethyl-3,3′-azoisooxazole; triphenylphosphine In acetonitrile at 80℃; Mitsunobu reaction; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; Aliquat 336 1.) 120 deg C, 5 min; 2.) 85 deg C, 2h; | 99% |
| With sodium hydroxide In N,N-dimethyl acetamide at 65℃; Rate constant; also in KF-cryptand 222 system; | |
| With sodium hydroxide In N,N-dimethyl acetamide at 25℃; Rate constant; | |
| With methanol; potassium hydroxide under 200 Torr; | |
| With aluminum oxide; potassium fluoride In acetonitrile for 20h; | 8 % Chromat. |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
88-75-5
2-hydroxynitrobenzene

-
-
835-71-2
2-(4-methylphenyl)-1,3-benzoxazole

| Conditions | Yield |
|---|---|
| With formic acid In 1,4-dioxane; water at 80℃; for 8h; Sealed tube; Green chemistry; | 99% |
| Stage #1: 4-methyl-benzaldehyde; 2-hydroxynitrobenzene In 1,4-dioxane; water at 80℃; for 0.0833333h; Stage #2: With formic acid In 1,4-dioxane; water at 80℃; for 8h; Sealed tube; | 99% |
| With 1,4-diaza-bicyclo[2.2.2]octane; sulfur at 130℃; for 2h; | 87% |
| In tetralin |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
88-75-5
2-hydroxynitrobenzene

-
-
838-34-6
2-(p-methoxyphenyl)benzoxazole

| Conditions | Yield |
|---|---|
| With formic acid In 1,4-dioxane; water at 80℃; for 8h; Sealed tube; Green chemistry; | 99% |
| Stage #1: 4-methoxy-benzaldehyde; 2-hydroxynitrobenzene In 1,4-dioxane; water at 80℃; for 0.0833333h; Stage #2: With formic acid In 1,4-dioxane; water at 80℃; for 8h; Sealed tube; | 99% |
| With poly(ethylene glycol)-bound sulphonic acid In chloroform at 50 - 60℃; | 95% |
| With 1,4-diaza-bicyclo[2.2.2]octane; sulfur at 130℃; for 2h; | 82% |
| In tetralin |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone a) RT, 24 h, b) reflux, 120 h; | 99% |
-
-
16695-22-0
N,N-bis(tosyloxyethyl)-p-toluenesulfonamide

-
-
88-75-5
2-hydroxynitrobenzene

-
-
54533-67-4
1,5-bis(2-nitrophenoxy)-3-(4-toluenesulfonyl)-3-azapentane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 8h; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide | |
| With potassium carbonate In N,N-dimethyl-formamide for 2h; Heating; |
-
-
29263-94-3
diethyl bromomethylmalonate

-
-
88-75-5
2-hydroxynitrobenzene

-
-
188407-70-7
diethyl 2-methyl-2-(2'-nitrophenoxy)malonate

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxynitrobenzene With potassium hydroxide In ethanol for 0.5h; Stage #2: diethyl bromomethylmalonate In N,N-dimethyl-formamide for 16h; Inert atmosphere; | 99% |
| With potassium fluoride 1.) DMF, RT, 15 min, 2.) DMF, 60 deg C, 6 h; Yield given. Multistep reaction; |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
88-75-5
2-hydroxynitrobenzene

-
-
207444-89-1
(2-nitrophenoxy)-acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxynitrobenzene With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Stage #2: bromoacetic acid tert-butyl ester In tetrahydrofuran at 20℃; for 23h; Reflux; | 99% |
| With sodium hydride 1.) THF, RT, 0.5, 2.) THF, reflux, 7 d; Yield given. Multistep reaction; | |
| With caesium carbonate In acetone for 48h; Heating / reflux; |
-
-
685-87-0
Diethyl 2-bromomalonate

-
-
88-75-5
2-hydroxynitrobenzene

-
-
32539-24-5
diethyl 2-(2'-nitrophenoxy)malonate

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxynitrobenzene With potassium hydroxide In ethanol for 0.5h; Stage #2: Diethyl 2-bromomalonate In N,N-dimethyl-formamide for 16h; Inert atmosphere; | 99% |
| With potassium fluoride In N,N-dimethyl-formamide at 60℃; for 6h; Substitution; | 70% |
2-Nitrophenol Specification
The 2-Nitrophenol with CAS registry number of 88-75-5 is also known as Phenol, 2-nitro-. The IUPAC name and product name are the same. It belongs to product categories of Intermediates of Dyes and Pigments; Organics; Phenoles and thiophenoles; Analytical Chemistry; Indicator (pH); pH Indicators; Indicators; PH Indicators - Solids; Titration; Organic Building Blocks; Oxygen Compounds; Phenols; Alpha Sort; N; NA - NI; N-OAlphabetic; Volatiles/ Semivolatiles; N-PAlphabetic; Pesticides&Metabolites; Dye Intermediates. Its EINECS registry number is 201-857-5. In addition, the formula is C6H5NO3 and the molecular weight is 139.11.
Physical properties about 2-Nitrophenol are: (1)ACD/LogP: 1.71; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.7; (4)ACD/LogD (pH 7.4): 1.26; (5)ACD/BCF (pH 5.5): 11.45; (6)ACD/BCF (pH 7.4): 4.15; (7)ACD/KOC (pH 5.5): 198.06; (8)ACD/KOC (pH 7.4): 71.84; (9)#H bond acceptors: 4; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Index of Refraction: 1.612; (13)Molar Refractivity: 34.67 cm3; (14)Molar Volume: 99.7 cm3; (15)Surface Tension: 60.2 dyne/cm; (16)Density: 1.395 g/cm3; (17)Flash Point: 97.1 °C; (18)Enthalpy of Vaporization: 47.06 kJ/mol; (19)Boiling Point: 215.8 °C at 760 mmHg; (20)Vapour Pressure: 0.0987 mmHg at 25 °C.
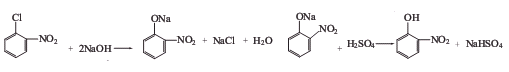
Preparation of 2-Nitrophenol: it is prepared by reaction of nitrochlorobenzene with sodium hydroxide and sulfuric acid. The reaction has two steps of hydrolysis and acidification.
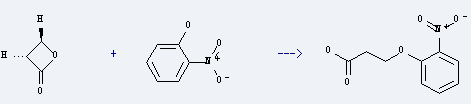
Uses of 2-Nitrophenol: it is used to produce 3-(2-nitro-phenoxy)-propionic acid by reaction with oxetan-2-one. The reaction occurs with reagent NaOH and other condition of heating for 40 minutes. The yield is about 37%. This chemical is used as organic synthesis intermediate of medicine, dyes, rubber chemicals, photographic materials and also can be used as color pH indicator.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. It's harmful and has danger of very serious irreversible effects by inhalation, in contact with skin and if swallowed. Besides, it is highly flammable and harmful to aquatic organisms that may cause long-term adverse effects in the aquatic environment. During using it, wear suitable protective clothing, gloves and eye/face protection. Keep away from sources of ignition and avoid release to the environment. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If accident happens or you feel unwell seek medical advice immediately. After using it, keep container tightly closed. After contact with skin, wash immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C(=C1)[N+](=O)[O-])O
2. InChI: InChI=1S/C6H5NO3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H
3. InChIKey: IQUPABOKLQSFBK-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | subcutaneous | 600mg/kg (600mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 120, 1959. | |
| dog | LD50 | intravenous | 100mg/kg (100mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 675, 1986. | |
| frog | LDLo | subcutaneous | 300mg/kg (300mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 120, 1959. | |
| guinea pig | LDLo | subcutaneous | 900mg/kg (900mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: COMA | Revue Medicale de la Suisse Romande. Vol. 16, Pg. 449, 1896. |
| mouse | LD50 | intraperitoneal | 378mg/kg (378mg/kg) | Journal of Medicinal Chemistry. Vol. 18, Pg. 868, 1975. | |
| mouse | LD50 | oral | 1297mg/kg (1297mg/kg) | Aerospace Medical Research Laboratory Report. Vol. TR-72-62, Pg. 1972, | |
| mouse | LDLo | intramuscular | 600mg/kg (600mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 120, 1959. | |
| rabbit | LD50 | skin | > 7940mg/kg (7940mg/kg) | BEHAVIORAL: FOOD INTAKE (ANIMAL) BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 74, 1990. |
| rabbit | LDLo | subcutaneous | 1700mg/kg (1700mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 120, 1959. | |
| rat | LD50 | intraperitoneal | 840mg/kg (840mg/kg) | BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: DYSPNEA KIDNEY, URETER, AND BLADDER: STRUCTURAL OR FUNCTIONAL CHANGES IN URETER | National Technical Information Service. Vol. OTS0545982, |
| rat | LD50 | oral | 334mg/kg (334mg/kg) | Gigiena Truda i Professional'naya Patologiya v Estonskoi SSR. Labor Hygiene and Occupational Pathology in the Estonian SSR. Vol. 8, Pg. 145, 1972. | |
| rat | LDLo | subcutaneous | 1100mg/kg (1100mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: COMA | Revue Medicale de la Suisse Romande. Vol. 16, Pg. 449, 1896. |
Related Products
- 2-Nitrophenol
- 887574-80-3
- 887576-89-8
- 887577-30-2
- 887577-86-8
- 887578-57-6
- 887578-93-0
- 887579-62-6
- 887580-83-8
- 887581-34-2
- 887581-41-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View