-
Name
2-Phenylacetamide
- EINECS 203-147-0
- CAS No. 103-81-1
- Article Data287
- CAS DataBase
- Density 1.098 g/cm3
- Solubility
- Melting Point 156 °C
- Formula C8H9NO
- Boiling Point 312.2 °C at 760 mmHg
- Molecular Weight 135.166
- Flash Point 142.6 °C
- Transport Information
- Appearance White krystalline powder.
- Safety 22-24/25
- Risk Codes 22-36
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acetamide,2-phenyl- (6CI,8CI);Benzenediacetamide (7CI);Benzeneacetamide;NSC 1877;Phenacetamide;Phenyl-b-acetylamine;Phenylacetic acid amide;a-Phenylacetamide;a-Toluamide;a-Toluimidic acid;
- PSA 43.09000
- LogP 1.41470
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide; trisodium tris(3-sulfophenyl)phosphine; water; chloro(1,5-cyclooctadiene)rhodium(I) dimer In ethyl acetate at 90℃; for 24h; pH=11.7; hydration; | 100% |
| With Acetaldehyde oxime In methanol at 65℃; for 4h; | 100% |
| With [RuH(tBu-PNP(-))(CO)]; water In tert-butyl alcohol at 20℃; for 24h; | 99% |
-
-
30516-21-3
1-(1H-benzo[d][1,2,3]triazol-1-yl)-2-phenylethan-1-one

-
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In tetrahydrofuran; ethanol at 20℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; ammonium carbamate In tetrahydrofuran at 100℃; for 24h; Reagent/catalyst; Inert atmosphere; Molecular sieve; | 99% |
| Stage #1: phenylacetic acid With niobium pentachloride In dichloromethane Stage #2: With ammonia In dichloromethane at 45 - 50℃; for 0.5h; | 93% |
| Stage #1: phenylacetic acid With thionyl chloride In chloroform at 0 - 70℃; for 3h; Stage #2: With ammonia In chloroform; water at 20℃; for 0.166667h; | 91.8% |
-
-
33054-04-5
phenylacetyl azide

-
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With (1,4-diazabicyclo{2.2.2}-octane)zinc(II) tetrahydoborate In tetrahydrofuran for 0.75h; Ambient temperature; | 98% |
-
-
1299492-08-2
2-[(R)-(carbamoylphenylmethyl)-amino]-3-(4-methoxyphenyl)-2-(S)-methylpropionamide

-
A
-
1299492-09-3
2-(S)-amino-3-(4-methoxyphenyl)-2-methylpropionamide

-
B
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on carbon In isopropyl alcohol for 1h; Product distribution / selectivity; Inert atmosphere; Reflux; | A 96% B n/a |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate at 130℃; for 0.5h; Temperature; Inert atmosphere; Microwave irradiation; | 96% |

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate; hydroxylamine hydrochloride; sodium acetate at 110℃; for 5h; Neat (no solvent); | 95% |
| Stage #1: phenylacetaldehyde With hydroxylamine hydrochloride; sodium carbonate In water at 20℃; for 0.5h; Schlenk technique; Stage #2: With [(eta.(5)-pentamethylcyclopentadienyl)Ir(H2O)3](OTf)2 In water at 110℃; for 12h; Schlenk technique; | 86% |
| Stage #1: phenylacetaldehyde With hydroxylamine hydrochloride; sodium carbonate In water at 20℃; for 0.5h; Schlenk technique; Stage #2: With [(eta.(5)-pentamethylcyclopentadienyl)Ir(H2O)3](OTf)2 In water at 110℃; for 12h; | 86% |

-
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In toluene for 6h; Product distribution; Further Variations:; Reagents; Temperatures; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; dihydrogen peroxide In isopropyl alcohol | 95% |

-
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With sodium at 140℃; for 5.5h; Autoclave; | 94.8% |
-
-
106266-43-7
N-phenyliodonio α-phenylacetamide tosylate

-
A
-
932-72-9
(Dichloroiodo)benzene

-
B
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water | A 94% B 67% |

| Conditions | Yield |
|---|---|
| With Zn(NO3)2 In n-heptane for 16h; Reflux; | 94% |
| With [(eta.(5)-pentamethylcyclopentadienyl)Ir(H2O)3](OTf)2 In water at 110℃; for 12h; Schlenk technique; | 92% |
| With [(eta.(5)-pentamethylcyclopentadienyl)Ir(H2O)3](OTf)2 In water at 110℃; for 12h; Schlenk technique; | 92% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane for 1h; Ambient temperature; | 93% |

| Conditions | Yield |
|---|---|
| With amino(methyl)aluminum chloride In benzene at 50℃; for 12h; | 90% |
| With ammonium hydroxide | |
| With ammonia | |
| With ammonia In ethanol; water Reflux; |
-
-
112403-78-8
N-phenylacetyl-O-methylhydroxylamine

-
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; acetic acid at 100℃; for 6h; | 90% |
| With titanium(III) chloride; air 1) H2O, EtOH, 3 h, 40 deg C; Yield given. Multistep reaction; |
-
-
1309977-13-6
C13H17N3O

-
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 0.166667h; | 90% |

| Conditions | Yield |
|---|---|
| With [1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride; magnesium chloride; zinc In N,N-dimethyl-formamide at 70℃; for 24h; | 90% |
-
-
65999-53-3
2-(2-bromophenyl)acetamide

-
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; cesium acetate; isopropyl alcohol at 100℃; Schlenk technique; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium periodite In dichloromethane at 20℃; for 0.116667h; | 89% |

| Conditions | Yield |
|---|---|
| With formamide at 100 - 120℃; Neat (no solvent); | 88% |
| With ammonium hydroxide | |
| With ammonium hydroxide In dichloromethane at 0 - 20℃; |
-
-
19227-11-3
N'-hydroxy-2-phenylethanimidamide

-
A
-
140-29-4
phenylacetonitrile

-
B
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In acetonitrile at 20℃; for 0.5h; | A 88% B 10% |
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In water; acetonitrile at 20℃; for 0.5h; | A 10% B 81% |
| With oxygen; sodium methylate In methanol at 20℃; Irradiation; | A 18% B 77% |

| Conditions | Yield |
|---|---|
| With [1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride; magnesium chloride; zinc In N,N-dimethyl-formamide at 70℃; for 24h; | 88% |

| Conditions | Yield |
|---|---|
| With [1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride; magnesium chloride; zinc In N,N-dimethyl-formamide at 70℃; for 24h; | 85% |

| Conditions | Yield |
|---|---|
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In water at 25℃; for 48h; regioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With Oxone; ammonium bicarbonate; [Mn(2,6-Cl2-TPP)Cl] In water; acetonitrile at 25℃; for 2h; | 84% |
| With pyridine; ammonium hydroxide; sulfur at 165℃; | |
| With ammonia; dinitrogen monoxide at 250℃; under 147102 Torr; |

| Conditions | Yield |
|---|---|
| With sodium methylate; formamide In N,N-dimethyl-formamide at 100℃; for 0.5h; | 84% |
| With ammonia In water at 25℃; for 24h; | 66% |
| With ammonium hydroxide In water at 20℃; for 16h; Inert atmosphere; | |
| With ammonium hydroxide In methanol at 20℃; for 15h; |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid Reflux; | 82% |

-
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| With ammonium carbonate In dimethyl sulfoxide at 25℃; for 6h; | 81% |

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
28861-34-9
phenylacetylcarbamic acid methyl ester

-
B
-
103-81-1
Benzeneacetamide

| Conditions | Yield |
|---|---|
| In toluene for 12h; Heating; | A 80% B n/a |


| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In methanol for 0.166667h; Heating; | 100% |
| With methanol; bromine |

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; mercury(II) diacetate In N,N-dimethyl-formamide for 12h; Ambient temperature; | 100% |
| Stage #1: Benzeneacetamide With 2,3,4,5,6-pentamethyliodobenzene; oxygen; isobutyraldehyde In 1,2-dichloro-ethane at 40℃; under 760.051 Torr; for 3h; Hofmann Rearrangement; Stage #2: methanol In 1,2-dichloro-ethane for 24h; Hofmann Rearrangement; | 100% |
| With iodobenzene; oxone at 20℃; Hofmann rearrangement; | 97% |
-
-
103-81-1
Benzeneacetamide

-
-
3287-99-8, 39110-74-2
benzylamine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: Benzeneacetamide With tetrafluoroboric acid; iodobenzene; 3-chloro-benzenecarboperoxoic acid In water; acetonitrile at 20℃; for 2h; Hofmann rearrangement; Inert atmosphere; Stage #2: With hydrogenchloride In water; acetonitrile Inert atmosphere; | 100% |
| With hydrogenchloride; formic acid; iodosylbenzene 1.) CH3CN, H2O, 15 h, room temp.; Yield given. Multistep reaction; |

-
-
103-81-1
Benzeneacetamide

-
A
-
14613-34-4
benzylamine p-toluenesulfonic acid

-
B
-
144970-30-9
1,3,5,7-tetrakis(4-iodophenyl)adamantane

| Conditions | Yield |
|---|---|
| In acetonitrile for 1h; Heating; | A 82% B 100% |

-
-
103-81-1
Benzeneacetamide

-
-
88333-03-3, 10340-91-7
Benzyl isocyanide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; trichlorophosphate at -10℃; | 100% |
| With pyridine; methanesulfonyl chloride In toluene at 35 - 40℃; for 1h; |

| Conditions | Yield |
|---|---|
| Stage #1: Benzeneacetamide With borane tetrahydrofuran In toluene at 0 - 20℃; for 1h; Stage #2: leelamine In toluene Reflux; | 99.68% |

| Conditions | Yield |
|---|---|
| Stage #1: Benzeneacetamide With hydrogenchloride; water Heating; Stage #2: With sodium hydroxide Reagent/catalyst; | 99.5% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water 1) 70 deg C, 5 min; 2) 25 deg C, overnight; | 99% |
| With aluminum oxide; water at 57 - 60℃; for 0.0333333h; microwave irradiation; | 70% |

| Conditions | Yield |
|---|---|
| With triethyl borane; phenylsilane; potassium acetate In tetrahydrofuran; tert-butyl methyl ether at 20℃; for 48h; Reagent/catalyst; Temperature; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | 99% |
| With triethyl borane; phenylsilane; potassium acetate In tetrahydrofuran; tert-butyl methyl ether at 20℃; for 48h; Reagent/catalyst; Temperature; Inert atmosphere; Schlenk technique; Glovebox; Sealed tube; | 99% |
| With allyl bromide; 1,1'-carbonyldiimidazole In acetonitrile 1.) 0.5 h, RT; 2.) 2 h, reflux; | 98% |
-
-
18372-22-0
2-(3-indolyl)oxoacetic acid methyl ester

-
-
103-81-1
Benzeneacetamide

-
-
125313-57-7
3-(3-indolyl)-4-phenyl-1H-pyrrole-2,5-dione

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; | 99% |
| With potassium tert-butylate | 99% |
| With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; |
-
-
51079-10-8
ethyl 2-(1H-indol-3-yl)-2-oxoacetate

-
-
103-81-1
Benzeneacetamide

-
-
125313-57-7
3-(3-indolyl)-4-phenyl-1H-pyrrole-2,5-dione

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; | 99% |
-
-
103-81-1
Benzeneacetamide

-
-
105-13-5
4-Methoxybenzyl alcohol

-
-
305849-49-4
N-(4-methoxybenzyl)-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) at 175℃; for 20h; Sealed tube; | 99% |
-
-
956-04-7
3-(4-chlorophenyl)-1-phenylprop-2-en-1-one

-
-
103-81-1
Benzeneacetamide

-
-
70028-38-5
4-(p-Chlorphenyl)-3,6-diphenyl-3,4-dihydro-2(1H)-pyridon

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 2h; Heating; | 98% |
-
-
13081-18-0
ethyl-3,3,3-trifluoropyruvate

-
-
103-81-1
Benzeneacetamide

-
-
126535-82-8
Ethyl 2-phenylacetylamino-2-hydroxy-3,3,3-trifluoropropionate

| Conditions | Yield |
|---|---|
| In dichloromethane for 2h; Ambient temperature; | 98% |
-
-
117-80-6
2,3-Dichloro-1,4-naphthoquinone

-
-
103-81-1
Benzeneacetamide

-
-
63351-46-2
N-(3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)phenylacetamide

| Conditions | Yield |
|---|---|
| With water at 20℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) at 175℃; for 20h; Sealed tube; | 98% |
| With barium trifluoromethanesulfonate In toluene for 18h; Reflux; Inert atmosphere; | 91% |
-
-
103-81-1
Benzeneacetamide

-
-
25939-33-7
phenylacetohydroxamoyl chloride

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid In methanol at 20℃; for 1h; | 97.5% |
| With trichloroisocyanuric acid In chloroform; acetone at 20℃; for 4h; | 90% |
| With trichloroisocyanuric acid In methanol |

| Conditions | Yield |
|---|---|
| With benzene-1,2-dicarboxylic acid at 250℃; under 7600 Torr; for 0.333333h; microwave irradiation; | 97% |
| With phthalic anhydride at 240 - 250℃; under 3040 Torr; for 0.75h; Hydrolysis; | 95% |
| With titanium tetrachloride In 1,4-dioxane; water for 17h; Heating; | 91% |
| With niobium(V) oxide; water In neat (no solvent) for 24h; Reflux; Inert atmosphere; | 88% |
| With cell-free extract of amidase gene cloned from Klebsiella oxytoca KCTC 1686 and functionally expressed in Escherichia coli BL21(DE3) In methanol Enzymatic reaction; |
-
-
553-90-2
Dimethyl oxalate

-
-
103-81-1
Benzeneacetamide

-
-
84863-93-4
3-hydroxy-4-phenyl-1H-pyrrole-2,5-dione

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In DMF (N,N-dimethyl-formamide) at 0 - 20℃; for 5h; | 97% |
| With potassium tert-butylate In N,N-dimethyl-formamide | |
| With potassium hydroxide In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With graphene oxide In neat (no solvent) at 130℃; for 20h; Sealed tube; | 97% |
| With 1-(3-sulfopropyl)pyridinium phosphotungstate In neat (no solvent) at 120℃; for 0.666667h; Microwave irradiation; | 91% |
| With sulfated tungstate In toluene for 12h; Reflux; Green chemistry; | 90% |
-
-
103-81-1
Benzeneacetamide

-
-
94-41-7
benzalacetophenone

-
-
70028-35-2
3,4,6-triphenyl-3,4-dihydro-1H-pyridin-2-one

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 2h; Heating; | 96% |

| Conditions | Yield |
|---|---|
| With acetic anhydride; toluene-4-sulfonic acid at 150℃; for 0.333333h; microwave irradiation; | 96% |
| With acetic anhydride; toluene-4-sulfonic acid In 1-methyl-pyrrolidin-2-one at 120℃; for 24h; | 58% |

-
-
4319-49-7
1-aminomorpholine

-
-
103-81-1
Benzeneacetamide

-
-
543686-21-1
N-(morpholin-4-yl)-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With Fe3+ exchanged montmorillonite K-10 In neat (no solvent) at 140℃; for 30h; Inert atmosphere; | 96% |
2-Phenylacetamide Chemical Properties
Structure of 2-Phenylacetamide (CAS NO.103-81-1):
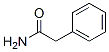
IUPAC Name: 2-phenylacetamide
Empirical Formula: C8H9NO
Molecular Weight: 135.1632
EINECS: 203-147-0
Index of Refraction: 1.552
Molar Refractivity: 39.36 cm3
Molar Volume: 123 cm3
Polarizability: 15.6×10-24cm3
Surface Tension: 44.5 dyne/cm
Density: 1.098 g/cm3
Flash Point: 142.6 °C
Enthalpy of Vaporization: 55.32 kJ/mol
Melting Point: 156 °C
Boiling Point: 312.2 °C at 760 mmHg
Vapour Pressure: 0.000537 mmHg at 25°C
Canonical SMILES: C1=CC=C(C=C1)CC(=O)N
InChI: InChI=1S/C8H9NO/c9-8(10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,9,10)
InChIKey: LSBDFXRDZJMBSC-UHFFFAOYSA-N
2-Phenylacetamide Uses
2-Phenylacetamide (CAS NO.103-81-1) are used as intermediates for penicillin G and phenobarbital drug , etc. It is also used in organic synthesis.
2-Phenylacetamide Toxicity Data With Reference
| 1. | ipr-mus LD50:430 mg/kg | PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN. 10 (1976),579. |
2-Phenylacetamide Consensus Reports
Reported in EPA TSCA Inventory.
2-Phenylacetamide Safety Profile
Moderately toxic by intraperitoneal route. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes:  Xi
Xi
Safety Statements: 22-24/25
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
RTECS: AC7705000
Hazard Note: Irritant
2-Phenylacetamide Specification
2-Phenylacetamide , its cas register number is 103-81-1. It also can be called Phenyl-beta-acetylamine ; Phenylacetic acid amide ; alpha-Phenylacetamide ; alpha-Toluamide ; alpha-Toluimidic acid .
Related Products
- 2-Phenylacetamide
- 103812-00-6
- 10381-36-9
- 10381-70-1
- 10381-75-6
- 103818-46-8
- 103818-83-3
- 103819-42-7
- 103819-44-9
- 103819-46-1
- 103-82-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View