-
Name
3-Bromobenzaldehyde
- EINECS 221-526-9
- CAS No. 3132-99-8
- Article Data224
- CAS DataBase
- Density 1.577 g/cm3
- Solubility decomposes in water
- Melting Point 18-21 °C(lit.)
- Formula C7H5BrO
- Boiling Point 235.3 °C at 760 mmHg
- Molecular Weight 185.02
- Flash Point 96.1 °C
- Transport Information
- Appearance clear colorless to yellow liquid after melting
- Safety 26-36
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms NSC 66828;m-Bromobenzaldehyde;Benzaldehyde,m-bromo- (6CI,7CI,8CI);3-Formyl-1-bromobenzene;3-Formylbromobenzene;
- PSA 17.07000
- LogP 2.26160
Synthetic route
-
-
15852-73-0
(3-Bromophenyl)methanol

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride; potassium carbonate; dimethyl sulfoxide at 20℃; for 12h; chemoselective reaction; | 99% |
| With N-chloro-N-(benzenesulfonyl)benzenesulfonamide In acetonitrile at 20 - 25℃; for 0.25h; Inert atmosphere; chemoselective reaction; | 98% |
| With tert.-butylhydroperoxide; 2,2′-((1E,1′E)-(1,2-phenylenebis(azanylylidene))bis(methanylylidene))diphenolcopper(II); sodium hydroxide In water; acetonitrile at 20℃; | 97% |
-
-
14473-91-7, 20595-41-9, 32862-97-8
3-bromocinnamic acid

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium permanganate In dichloromethane at 20℃; | 99% |
-
-
108-36-1
1,3-dibromobenzene

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dibromobenzene With tri-n-butyllithium magnesate complex In toluene at 0℃; for 1.5h; Stage #2: N,N-dimethyl-formamide In toluene at 0℃; for 0.5h; Stage #3: With citric acid at 20℃; | 99% |
| Stage #1: 1,3-dibromobenzene With n-butyllithium; isopropylmagnesium chloride In tetrahydrofuran; hexane at 0 - 5℃; for 1h; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at 0℃; for 1h; Further stages.; | 90% |
| Stage #1: 1,3-dibromobenzene With n-butyllithium In tetrahydrofuran at -78℃; for 0.583333h; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at -78 - 20℃; for 1.5h; | 63.21% |
-
-
62247-78-3
1-bromo-3-(dibromomethyl)benzene

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide at 120℃; for 1h; | 95% |
| With potassium carbonate In dimethylsulfoxide-d6 at 60℃; for 24h; Kinetics; Hydrolysis; |
-
-
75148-49-1
3-bromobenzaldehyde diethylacetal

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With iron(III) p-toluenesulfonate hexahydrate In water for 1h; Reflux; | 95% |
| bismuth(III) iodide In water at 20℃; for 20h; | 89% |
| With copper(II) sulfate; sodium iodide In acetone at 56℃; for 0.75h; | 77% |
-
-
62247-78-3
1-bromo-3-(dibromomethyl)benzene

-
-
67-68-5
dimethyl sulfoxide

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| at 120℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| With bromine fluoride In ethanol; chloroform at -40℃; for 0.0833333h; | A 94% B 5% |
| With bromine fluoride In ethanol; chloroform at -40℃; for 0.833333h; | A 5% B 90% |
-
-
82436-20-2
2-(3-bromophenyl)-1,3-dithiolane

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With silica gel; ferric nitrate In hexane at 50℃; for 0.166667h; | 94% |
-
-
50793-32-3
C6H4(3-Br)(CHSCH2CH2CH2S-cyclic)

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With silica gel; iodic acid at 20℃; for 0.0125h; | 94% |
| With quinolinium monofluorochromate(VI) In acetonitrile for 2.5h; Heating; | 93% |
| With 1,3,5-trichloro-2,4,6-triazine; dimethyl sulfoxide In dichloromethane at 20℃; for 2h; | 92% |
| With 2,6-dicarboxypyridinium chlorochromate In acetonitrile for 1.91667h; Heating; | 90% |
-
-
17789-14-9
3-(1,3-dioxolan-2-yl)-phenylbromide

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With carbon tetrabromide In water; acetonitrile at 45℃; for 2h; sonication; | 93% |
| In(OSO2CF3)3 In acetone at 20℃; for 2.5h; | 93% |
| With copper(II) sulfate; sodium iodide In acetone at 56℃; for 0.666667h; | 92% |
-
-
867371-13-9
1-bromo-3-(trimethylsilyloxy)methyl-benzene

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonium peroxodisulphate In acetonitrile for 0.2h; Reflux; | 93% |
| With ethylenebis(N-methylimidazolium) chlorochromate In acetonitrile for 5.5h; Reflux; | 85% |
| With trichloroisocyanuric acid In acetonitrile Reflux; |
-
-
38407-30-6
1-(3-bromobenzylidene)semicarbazide

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; water; silica gel In dichloromethane at 20℃; for 3.5h; | 92% |
-
-
643734-33-2
(3-bromo-phenyl)-pyrrol-1-yl-methanol

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 20℃; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| With Selectfluor In acetonitrile at 20℃; for 0.333333h; | 90% |
| With [bis(acetoxy)iodo]benzene; sodium hydrogencarbonate In chloroform at 60℃; for 12h; Polonovski type reaction; | 42% |

| Conditions | Yield |
|---|---|
| With calcium bis(hypophosphite); calcium acetate; nickel(II) acetate tetrahydrate In ethanol; water at 100℃; for 29h; Sealed tube; | 90% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride hexahydrate In acetonitrile at 100℃; Sealed tube; | A 76% B 90% |

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In methanol at -10 - 10℃; | 89% |
-
-
51873-95-1, 52739-46-5, 32605-62-2
3-bromobenzaldehyde oxime

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With samarium; titanium tetrachloride In tetrahydrofuran at 20℃; for 0.166667h; | 88% |
| With chromium(VI) oxide; silica gel In toluene at 72 - 75℃; for 3h; Oxidation; Deoximation; | 86% |
| With manganese(IV) oxide; silica gel for 0.1h; microwave irradiation; | 86% |

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With N-sulfonic acid poly(4-vinylpyridinium) chloride In methanol at 20℃; for 0.583333h; | 88% |
| With sulfonated rice husk ash In acetonitrile at 60℃; for 0.0833333h; | 88% |
| With water at 20℃; for 0.25h; Green chemistry; | 93 %Chromat. |

| Conditions | Yield |
|---|---|
| With oxygen; copper diacetate In dimethyl sulfoxide at 120℃; for 18h; Sealed tube; | 87% |
| With (2,2'-dipyridyl)bis(5-methyl-2-(4-fluoro)phenylpyridine-N,C)iridium(III) hexafluorophosphate; oxygen; sodium carbonate; 1,1'-diethyl-4,4'-bipyridinium diperchlorate In dimethyl sulfoxide at 20℃; under 760.051 Torr; for 16h; Irradiation; | 71% |

| Conditions | Yield |
|---|---|
| With bis-{4-methoxy-phenyl}-selenoxyde; sodium hydrogencarbonate In acetonitrile at 75℃; for 5h; | 86% |
| With chloroform; hexamethylenetetramine Kochen des Reaktionsprodukts mit wss. Essigsaeure; | |
| With hydrogenchloride; ethanol; hexamethylenetetramine | |
| Multi-step reaction with 2 steps 1: water / 5 h / Reflux 2: dihydrogen peroxide / water / 7 h / 20 °C View Scheme | |
| With 4-methylmorpholine N-oxide at 20℃; for 3h; Reflux; | 259 mg |

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; oxygen In acetonitrile at 20℃; for 3h; Irradiation; Green chemistry; | 85% |
| With oxygen In water at 150℃; under 30002.4 Torr; for 2h; | 49% |
| With N-hydroxyphthalimide; oxygen; trifluoroacetic acid; sodium nitrite In water at 20℃; for 3.5h; Green chemistry; | 13% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; sulfuric acid; trifluoroacetic acid at 25℃; for 42h; Bromination; | 84% |
| With potassium hydrogensulfate; isoquinolinium dichromate; potassium bromide In water at 20℃; Reagent/catalyst; Temperature; Sonication; regioselective reaction; | 72% |
| With monobromoisocynaurate; sulfuric acid at 40℃; for 12h; | 70% |

| Conditions | Yield |
|---|---|
| With trifuran-2-yl-phosphane; palladium diacetate In tetrahydrofuran; toluene at 0℃; for 0.5h; Schlenk technique; | 83% |

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In toluene at 80℃; Inert atmosphere; Sealed tube; | 83% |

| Conditions | Yield |
|---|---|
| With 1,2-bis(2,4,8,10-tetra-tert-butyl-5,7-dioxa-6-phosphadibenzo[a,c]cyclohepten-6-yloxy)ethane; methylphenylsilane; copper(l) cyanide; 2,2-dimethylpropanoic anhydride In dimethyl sulfoxide at 80℃; for 20h; Schlenk technique; Inert atmosphere; | 82% |
-
-
18257-89-1
1-allyl-3-bromo-benzene

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With iron(III) chloride; tetramethyldisiloxane In ethanol at 20℃; for 12h; | 82% |

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride trihydrate; hydrogen; triethylamine; triphenylphosphine In N,N-dimethyl acetamide at 90℃; under 7500.75 Torr; for 12h; Autoclave; | 82% |

| Conditions | Yield |
|---|---|
| With n-butyllithium; acetic acid In tetrahydrofuran; hexane; N,N-dimethyl-formamide; toluene | 78% |
-
-
337535-82-7
N-acetyl-3-bromobenzylamine

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In chloroform at 20℃; for 18h; | 78% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
925-90-6
ethylmagnesium bromide

-
-
74157-47-4
(+/-)-1-(3-bromophenyl)propanol

| Conditions | Yield |
|---|---|
| In diethyl ether at -78 - 20℃; | 100% |
| In diethyl ether | 92% |
| Stage #1: m-bromobenzoic aldehyde; ethylmagnesium bromide In diethyl ether for 1.08333h; Cooling with ice; Stage #2: With hydrogenchloride In diethyl ether; water | 88% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
51873-95-1, 52739-46-5, 32605-62-2
3-bromobenzaldehyde oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water Reflux; | 100% |
| With pyridine; hydroxylamine hydrochloride for 2h; Reflux; | 91.5% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol at 45℃; for 1h; | 90.8% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
109-77-3
malononitrile

-
-
2972-74-9
2-(3-bromobenzylidene)malononitrile

| Conditions | Yield |
|---|---|
| With glycine In dichloromethane | 100% |
| With ethanolamine-phosphoric acid functionalized polyacrylonitrile fibers, hydrophobized with benzylamine (Bn-PANEAPF) In water at 20℃; for 2h; Knoevenagel Condensation; Green chemistry; | 99% |
| In various solvent(s) at 50℃; for 3h; Knoevenagel condensation; | 98% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
1066-54-2
trimethylsilylacetylene

-
-
77123-55-8
3-((trimethylsilyl)ethynyl)benzaldehyde

| Conditions | Yield |
|---|---|
| With copper(l) iodide; bis(benzonitrile)palladium(II) dichloride; tri-tert-butyl phosphine; diisopropylamine In 1,4-dioxane Inert atmosphere; | 100% |
| With copper(l) iodide; bis(benzonitrile)palladium(II) dichloride; tri-tert-butyl phosphine; diisopropylamine In 1,4-dioxane for 7h; Inert atmosphere; | 100% |
| With palladium diacetate; triethylamine; triphenylphosphine at 100℃; for 6h; Inert atmosphere; | 95% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
504-63-2
trimethyleneglycol

-
-
67437-93-8
2-(3-bromophenyl)-[1,3]dioxane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 15.5h; Reflux; Dean-Stark; | 100% |
| With toluene-4-sulfonic acid In toluene for 2.5h; Heating; | 99% |
| With toluene-4-sulfonic acid In toluene at 20℃; for 2.5h; | 96% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
26138-64-7
3-acetyl-2,4-dihydroxyquinoline

| Conditions | Yield |
|---|---|
| 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
107-21-1
ethylene glycol

-
-
17789-14-9
3-(1,3-dioxolan-2-yl)-phenylbromide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 5.5h; Heating / reflux; | 100% |
| With toluene-4-sulfonic acid In toluene at 20 - 135℃; | 98% |
| Stage #1: m-bromobenzoic aldehyde; ethylene glycol With toluene-4-sulfonic acid In toluene for 4h; Heating / reflux; Stage #2: With sodium hydrogencarbonate In water; toluene at 0℃; | 97% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
24455-93-4
N-(4-methoxyphenyl)ethane-1,2-diamine

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 1h; Condensation; | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
6332-77-0
N-(4-nitrophenyl)ethylenediamine

-
-
250386-71-1
N-[1-(3-Bromo-phenyl)-meth-(E)-ylidene]-N'-(4-nitro-phenyl)-ethane-1,2-diamine

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 1h; Condensation; | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
50622-50-9
N-(p-methylphenyl)-ethylenediamine

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 1h; Condensation; | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 1h; Condensation; | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
14088-83-6
N-(3-chlorophenyl)-1,2-diaminoethane

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 1h; Condensation; | 100% |

| Conditions | Yield |
|---|---|
| With gold(III) tribromide In water at 100℃; for 12h; | 100% |
| Stage #1: piperidine; m-bromobenzoic aldehyde With magnetic nanoparticles supported biimidazole Cu(I) complex for 0.166667h; Green chemistry; Stage #2: phenylacetylene In water for 1.5h; Reflux; Green chemistry; | 98% |
| With gold(III) immobilized magnetic poly imidazole and imidazolium bromide nanoparticles In water at 60℃; for 13h; Green chemistry; | 92% |


| Conditions | Yield |
|---|---|
| Stage #1: 5-methyl-2-hydroxyacetophenone With sodium hydroxide In ethanol; water at 0 - 5℃; Stage #2: m-bromobenzoic aldehyde In ethanol; water | 100% |
| With potassium hydroxide In ethanol at 20℃; for 24h; | |
| Stage #1: 5-methyl-2-hydroxyacetophenone With sodium hydroxide In methanol; water at 20℃; for 0.5h; Claisen Condensation; Stage #2: m-bromobenzoic aldehyde In methanol; water at 20℃; for 4h; Claisen Condensation; |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
118-93-4
o-hydroxyacetophenone

-
-
3754-50-5, 108440-17-1
3-(3-bromophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one

| Conditions | Yield |
|---|---|
| Stage #1: o-hydroxyacetophenone With sodium hydroxide In ethanol; water at 0 - 5℃; Stage #2: m-bromobenzoic aldehyde In ethanol; water | 100% |
| With sodium hydroxide In ethanol at 20℃; | 93% |
| With potassium hydroxide In ethanol at 20℃; for 24h; |
-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
151-50-8
potassium cyanide

-
-
71412-88-9, 550313-02-5
2-(3-bromophenyl)-2-hydroxyacetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: m-bromobenzoic aldehyde With sodium hydrogensulfite In water at 20℃; for 2.5h; Stage #2: potassium cyanide In water at 0 - 20℃; for 2h; | 100% |
| With acetic acid In methanol at 0℃; for 1h; |
-
-
913815-80-2
9-(4-aminobutyl)-9-azabicyclo[3.3.1]nonan-3α-yl 2-methoxy-5-methylphenylcarbamate

-
-
3132-99-8
m-bromobenzoic aldehyde

| Conditions | Yield |
|---|---|
| In benzene at 90℃; for 2h; | 100% |
| In benzene at 90℃; for 2h; |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
107-11-9
1-amino-2-propene

-
-
246509-64-8
Allyl-[1-(3-bromo-phenyl)-meth-(E)-ylidene]-amine

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 20h; | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
36282-40-3
(3-methoxyphenyl)magnesium bromide

-
-
76778-33-1
3-bromo-α-(3-methoxyphenyl)benzenemethanol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.25h; | 100% |
| Stage #1: m-bromobenzoic aldehyde; (3-methoxyphenyl)magnesium bromide In tetrahydrofuran at 80℃; for 3h; Grignard reaction; Inert atmosphere; Stage #2: With water; sodium chloride In tetrahydrofuran at 20℃; |
-
-
1122-62-9
2-acetylpyridine

-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
29816-74-8
(E)-3-(3-bromophenyl)-1-phenyl-2-propen-1-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
17789-14-9
3-(1,3-dioxolan-2-yl)-phenylbromide

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In ethylene glycol; toluene | 100% |
| With sodium hydroxide In ethylene glycol; toluene | 99% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
204191-43-5
racemic 3-amino-4,4-dimethyl-pentanoic acid

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 100% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
75-05-8
acetonitrile

-
-
1075178-25-4
3-(3-bromophenyl)-3-hydroxypropanenitrile

| Conditions | Yield |
|---|---|
| Stage #1: acetonitrile With potassium tert-butylate In tetrahydrofuran at -50℃; for 0.416667h; Inert atmosphere; Stage #2: m-bromobenzoic aldehyde In tetrahydrofuran at -40 - -10℃; | 100% |
| Stage #1: acetonitrile With potassium tert-butylate In tetrahydrofuran at -50℃; for 0.583333h; Inert atmosphere; Stage #2: m-bromobenzoic aldehyde In tetrahydrofuran at -50℃; for 0.583333h; Stage #3: With ammonium chloride In tetrahydrofuran; water | 96% |
| Stage #1: acetonitrile With potassium tert-butylate In tetrahydrofuran at -50℃; for 0.583333h; Stage #2: m-bromobenzoic aldehyde In tetrahydrofuran at -50 - 0℃; | 96% |
| Stage #1: acetonitrile With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.25h; Stage #2: m-bromobenzoic aldehyde In tetrahydrofuran at -78 - 20℃; Product distribution / selectivity; | 81% |
| Stage #1: acetonitrile With potassium tert-butylate In tetrahydrofuran at -50℃; for 0.5 - 0.583333h; Stage #2: m-bromobenzoic aldehyde In tetrahydrofuran at -50 - 20℃; for 3.5h; Product distribution / selectivity; | 69% |
3-Bromobenzaldehyde Chemical Properties
Synonyms: m-bromo-benzaldehyd;nsc66828; 3-bromo-benzaldehyd;AKOS BBS-00003144;Benzaldehyde, m-bromo-;3-BROMOBENZALDEHYDE;AKOS B004042;benzaldehyde,3-bromo-;
The Molecular formula of 3-bromo benzaldehyde(3132-99-8): C7H5BrO
The Molecular Weight of 3-bromo benzaldehyde(3132-99-8): 185.02
Molecular Structure :
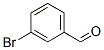
EINECS: 221-526-9
Melting point: 18-21 °C(lit.)
Boiling point: 233-236 °C(lit.)
density: 1.587 g/mL at 25 °C(lit.)
refractive index: n20/D 1.593(lit.)
Flash point: 96 °C
color: very faintly to deep brownish-yellow
Water Solubility: DECOMPOSES
Sensitive: Air Sensitive
3-Bromobenzaldehyde Safety Profile
 Xn,Xi
Xn,Xi The Risk Statements information of 3-bromo benzaldehyde(3132-99-8):
22: Harmful if swallowed
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of 3-bromo benzaldehyde(3132-99-8):
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
RIDADR: 2810
WGK Germany: 3
RTECS: CU4820000
F: 10
Hazard Note: Irritant
Related Products
- 3-Bromobenzaldehyde
- 3-Bromobenzaldehyde diethyl acetal
- 3133-07-1
- 31331-53-0
- 31333-13-8
- 313339-35-4
- 313339-36-5
- 313339-92-3
- 313348-27-5
- 313350-36-6
- 31335-33-8
- 313371-32-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View