-
Name
3-Methylanthranilic acid
- EINECS 224-505-2
- CAS No. 4389-45-1
- Article Data51
- CAS DataBase
- Density 1.254 g/cm3
- Solubility
- Melting Point 174-177 °C(lit.)
- Formula C8H9NO2
- Boiling Point 315.1 °C at 760 mmHg
- Molecular Weight 151.165
- Flash Point 144.4 °C
- Transport Information
- Appearance pink to grey-brown crystalline powder
- Safety 24/25-36-26
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms m-Toluicacid, 2-amino- (6CI,7CI,8CI);2-Amino-3-methylbenzoic acid;2-Amino-m-toluicacid;3-Methyl-2-aminobenzoic acid;
- PSA 63.32000
- LogP 1.85660
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol at 20℃; under 760 Torr; | 99% |
| With hydrogen; palladium on activated charcoal In ethanol at 20℃; under 25857.4 Torr; | 99% |
| With palladium 10% on activated carbon; hydrogen In ethanol at 20℃; under 760.051 Torr; | 99% |
-
-
15068-35-6
2-chloro-3-methylbenzoic acid

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| With ammonia; sodium carbonate; copper(l) chloride In dimethyl sulfoxide at 130 - 150℃; for 5h; | 93.1% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide | 90% |
| With potassium chloride; dihydrogen peroxide; sodium hydroxide In water at 0 - 20℃; for 2.25h; | 80% |
| 60% |
-
-
50424-93-6
3-methyl-2-nitrobenzoyl chloride

-
-
83325-79-5
methyl 3-methyl-N-methyl-N-(3-methyl-2-aminobenzoyl)anthranilate

-
A
-
4389-45-1
3-methylantranilic acid

-
B
-
83334-85-4
methyl 3-methyl-N-methyl-N-<3-methyl-N-(3-methyl-2-nitrobenzoyl)anthraniloyl>anthranilate

| Conditions | Yield |
|---|---|
| With lithium hydroxide In water; benzene | A n/a B 61% |

-
-
857203-66-8
8-methyl-3-o-tolyl-1H-quinazoline-2,4-dione

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
67-56-1
methanol

-
-
1127-59-9
7-methyl-1H-indole-2,3-dione

-
A
-
22223-49-0
methyl 2-amino-3-methylbenzoate

-
B
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium methylate 1.) 0-10 deg C 2.) room temperature, 30 min; Yield given. Multistep reaction; |

-
-
1127-59-9
7-methyl-1H-indole-2,3-dione

-
-
71-36-3
butan-1-ol

-
A
-
4389-45-1
3-methylantranilic acid

-
B
-
79101-84-1
3-Methylanthranilsaeure-n-butylester

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; sodium hydride 1.) 0-10 deg C 2.) room temperature, 30 min; Yield given. Multistep reaction; |


| Conditions | Yield |
|---|---|
| (i) H2, Pd-C, (ii) aq. NaOH; Multistep reaction; |
-
-
50424-93-6
3-methyl-2-nitrobenzoyl chloride

-
-
78877-00-6
3-methyl-N-methyl-N-(3-methyl-2-aminobenzoyl)anthranilic acid

-
A
-
4389-45-1
3-methylantranilic acid

-
B
-
83325-80-8
3-methyl-N-methyl-N-<3-methyl-N-(3-methyl-2-nitrobenzoyl)anthraniloyl>anthranilic acid

| Conditions | Yield |
|---|---|
| With lithium hydroxide In water; benzene Title compound not separated from byproducts; |

-
-
1127-59-9
7-methyl-1H-indole-2,3-dione

-
-
7722-84-1
dihydrogen peroxide

-
-
4389-45-1
3-methylantranilic acid

-
-
857203-66-8
8-methyl-3-o-tolyl-1H-quinazoline-2,4-dione

-
A
-
4389-45-1
3-methylantranilic acid

-
B
-
95-53-4
o-toluidine



-
-
7647-01-0
hydrogenchloride

-
-
5437-38-7
3-methyl-2-nitrobenzoic acid

-
A
-
4389-45-1
3-methylantranilic acid

-
B
-
95-53-4
o-toluidine


| Conditions | Yield |
|---|---|
| at 100℃; im Rohr; |
-
-
859770-30-2
(2-amino-3-methyl-phenyl)-glyoxylic acid

-
-
7722-84-1
dihydrogen peroxide

-
-
4389-45-1
3-methylantranilic acid

-
-
859770-30-2
(2-amino-3-methyl-phenyl)-glyoxylic acid

-
-
4389-45-1
3-methylantranilic acid


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 46 percent / HNO3 / 1 h / -10 °C 2: 99 percent / H2 / Pd/C / ethanol / 20 °C / 760 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: fuming nitric acid 2: tin; hydrochloric acid View Scheme | |
| Multi-step reaction with 2 steps 1: fuming nitric acid 2: tin; hydrochloric acid View Scheme | |
| Multi-step reaction with 2 steps 1: nitric acid / -10 - -5 °C 2: palladium 10% on activated carbon; hydrogen / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: nitric acid / 1 h / -10 °C 2: palladium 10% on activated carbon; hydrogen / ethanol / 2 h / 50 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 49 percent / fuming nitric acid / 1 h / -10 °C 2: 89 percent / tin, hydrochloric acid / ethanol / 1.5 h / 70 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 86percent H2SO4 / 1) 55 deg C, 10 min, 2) 75 deg C, 30 min 2: H2O2, NaOH View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid / 0.33 h / 80 °C 2: dihydrogen peroxide; sodium hydroxide / water / 0 - 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: sulfuric acid / 60 - 80 °C 2.1: dihydrogen peroxide; sodium hydroxide / water / 0.5 h / 0 - 50 °C 2.2: pH 4 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: concentrated hydrochloric acid / im Druckrohr 2: fuming nitric acid 3: tin; hydrochloric acid View Scheme |
-
-
60310-07-8
3-Methyl-2-nitrobenzenecarboxamide

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: POCl3 2: (i) H2, Pd-C, (ii) aq. NaOH View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium sulfate / water / 40 °C 1.2: Reflux 2.1: sulfuric acid / 0.33 h / 80 °C 3.1: dihydrogen peroxide; sodium hydroxide / water / 0 - 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium sulfate / water / 40 °C 1.2: 0.17 h / Reflux 2.1: sulfuric acid / 60 - 80 °C 3.1: dihydrogen peroxide; sodium hydroxide / water / 0.5 h / 0 - 50 °C 3.2: pH 4 View Scheme | |
| Multi-step reaction with 3 steps 1: hydrogenchloride; hydroxylamine hydrochloride; sodium sulfate / water / 55 °C 2: sulfuric acid / neat (no solvent) / 55 - 80 °C 3: sodium hydroxide; dihydrogen peroxide; potassium chloride / water / 2.25 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: sodium sulfate; hydrogenchloride; hydroxylamine hydrochloride / water / 0.17 h / Reflux 2: sulfuric acid / 0.83 h / 70 - 80 °C 3: dihydrogen peroxide; sodium hydroxide / water / 0.5 h / 0 - 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1: hydroxylamine hydrochloride; sodium sulfate; hydrogenchloride / water / 12 h / 55 °C / Inert atmosphere 2: sulfuric acid / 0.67 h / Heating; Inert atmosphere 3: dihydrogen peroxide; sodium hydroxide / water / 5.5 h / 80 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With Pseudomonas fluorescens kynureninase In aq. phosphate buffer at 37℃; pH=8; Kinetics; Enzymatic reaction; |
-
-
582319-05-9
ethyl 7-chloroindole-3-(3-acetamido-3-carboethoxy)butanoate

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sulfuric acid / water / 2 h / 55 - 60 °C 2: ozone / methanol / 1.5 h / 785.91 Torr / Cooling with acetone-dry ice 3: hydrogenchloride / water / 4 h / Reflux 4: Pseudomonas fluorescens kynureninase / aq. phosphate buffer / 37 °C / pH 8 / Enzymatic reaction View Scheme |

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ozone / methanol / 1.5 h / 785.91 Torr / Cooling with acetone-dry ice 2: hydrogenchloride / water / 4 h / Reflux 3: Pseudomonas fluorescens kynureninase / aq. phosphate buffer / 37 °C / pH 8 / Enzymatic reaction View Scheme |
-
-
1446522-63-9
ethyl 2-acetamido-2-carboethoxy-5-oxo-5-(2-formamido-3-methylphenyl)pentanoate

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water / 4 h / Reflux 2: Pseudomonas fluorescens kynureninase / aq. phosphate buffer / 37 °C / pH 8 / Enzymatic reaction View Scheme |
-
-
22223-49-0
methyl 2-amino-3-methylbenzoate

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; ethanol; water at 50℃; for 3h; Inert atmosphere; |
-
-
4389-45-1
3-methylantranilic acid

-
-
57772-50-6
2-amino-3-methylbenzyl alcohol

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylantranilic acid With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 2h; Stage #2: With water; sodium hydroxide In tetrahydrofuran | 100% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 2h; | 100% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 3.5h; | 97% |
-
-
4389-45-1
3-methylantranilic acid

-
-
206548-13-2
2-amino-5-bromo-3-methylbenzoic acid

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 20℃; for 1h; | 100% |
| With bromine; acetic acid at 20 - 25℃; | 97% |
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 70 - 80℃; for 0.5h; | 95.6% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylantranilic acid With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 0.666667h; Stage #2: methyl iodide In N,N-dimethyl-formamide at 20℃; | 100% |
| With caesium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; for 18.5h; | 92% |
| Stage #1: 3-methylantranilic acid With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: methyl iodide In N,N-dimethyl-formamide for 24h; | 77% |
| Stage #1: 3-methylantranilic acid With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: methyl iodide In N,N-dimethyl-formamide at 20℃; for 24h; | |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 18h; |
-
-
3786-39-8
3-carboxyphthalic anhydride

-
-
4389-45-1
3-methylantranilic acid

-
-
1158834-44-6, 1159266-95-1, 1159266-96-2
C17H11NO6

| Conditions | Yield |
|---|---|
| at 150℃; for 12h; | 100% |
-
-
1221280-89-2
6-[(R)-5-(3-amino-propyl)-2-oxo-oxazolidin-3-yl]-4H-benzo[1,4]thiazin-3-one

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| 100% |
-
-
4389-45-1
3-methylantranilic acid

-
-
20776-67-4
2-amino-5-chloro-3-methylbenzoic acid

| Conditions | Yield |
|---|---|
| With chlorine In 1,2-dichloro-ethane at 50℃; for 3h; Solvent; | 98.1% |
| With N-chloro-succinimide In N,N-dimethyl-formamide at 70 - 80℃; for 0.5h; | 97.8% |
| With N-chloro-succinimide In N,N-dimethyl-formamide at 75℃; | 91% |
-
-
4389-45-1
3-methylantranilic acid

-
-
118-91-2
ortho-chlorobenzoic acid

-
-
80841-45-8
2-<(2-carboxyphenyl)amino>-3-methylbenzoic acid

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate; copper(I) bromide In various solvent(s) at 160℃; for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In N,N-dimethyl-formamide; acetonitrile at 20℃; for 16h; | 98% |

| Conditions | Yield |
|---|---|
| 98% |
-
-
4389-45-1
3-methylantranilic acid

-
-
129-64-6
3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride

-
-
698393-13-4
C17H15NO4

| Conditions | Yield |
|---|---|
| at 110℃; for 16h; Inert atmosphere; neat (no solvent); | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylantranilic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 70℃; for 1h; Inert atmosphere; Stage #2: With ammonium hydroxide In N,N-dimethyl-formamide | 98% |
| Stage #1: 3-methylantranilic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 70℃; for 1h; Inert atmosphere; Stage #2: With ammonium hydroxide In N,N-dimethyl-formamide for 16h; Inert atmosphere; | 98% |
| Stage #1: 3-methylantranilic acid With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 70℃; for 1h; Inert atmosphere; Stage #2: With ammonium hydroxide In N,N-dimethyl-formamide for 16h; Inert atmosphere; | 98% |
-
-
14223-48-4
p-chlorophenyl isoselenocyanate

-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 40℃; for 4.5h; | 98% |
-
-
4389-45-1
3-methylantranilic acid

-
-
108857-24-5
2-amino-5-iodo-3-methylbenzoic acid

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; iodine In tetrachloromethane at 77℃; for 6h; | 97.7% |
| With N-iodo-succinimide In N,N-dimethyl-formamide at 20 - 80℃; for 2h; | 97% |
| With N-iodo-succinimide In N,N-dimethyl-formamide at 75℃; for 1h; Inert atmosphere; | 96% |
-
-
4389-45-1
3-methylantranilic acid

-
-
57024-47-2, 108146-85-6, 19634-85-6
6,6'-dimethyl-1,1'-biphenyl-2,2'-dicarboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylantranilic acid With hydrogenchloride; sodium nitrite In water at 0 - 5℃; for 0.5h; Stage #2: With copper(I) In water | 97% |
| With Cu(I)-salt; sodium nitrite | 78% |
| Stage #1: 3-methylantranilic acid With sodium hydroxide; sodium nitrite at 0℃; for 0.5h; Stage #2: With hydroxyammonium sulfate; copper(II) sulfate In water | 72% |
-
-
4389-45-1
3-methylantranilic acid

-
-
189999-35-7
[1,1'-biphenyl]-2,2'-iodonium trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 1,10-Phenanthroline; sodium carbonate In 1,4-dioxane at 95℃; for 17h; Molecular sieve; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; benzene Ambient temperature; | 96% |
| With sodium hydrogencarbonate In toluene at 0 - 20℃; for 0.25h; | 86% |
-
-
4389-45-1
3-methylantranilic acid

-
-
14702-32-0
α,α-diphenylsuccinic acid anhydride

-
-
297757-12-1, 297757-33-6, 297757-34-7
2-(2,5-dioxo-3,3-diphenylpyrrolidin-1-yl)-3-methylbenzoic acid

| Conditions | Yield |
|---|---|
| at 150℃; for 12h; Condensation; | 96% |
| at 150℃; neat (no solvent, solid phase); |

| Conditions | Yield |
|---|---|
| In formamide at 160℃; for 12h; | 96% |
| With formamide at 160℃; | 96% |
-
-
4389-45-1
3-methylantranilic acid

-
-
154592-60-6
1-fluoro-4-isoselenocyanatobenzene

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 40℃; for 4.5h; | 96% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 6h; Reflux; | 96% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
4389-45-1
3-methylantranilic acid

-
-
66176-17-8
6-methylisatoic anhydride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 6.5h; Reflux; | 95% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran; dichloromethane | 94% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran; dichloromethane | 94% |
-
-
4389-45-1
3-methylantranilic acid

-
-
13149-00-3
1,2-cis-cyclohexanedicarboxylic anhydride

-
-
1185155-84-3
C16H17NO4

| Conditions | Yield |
|---|---|
| at 110℃; for 10h; Inert atmosphere; neat (no solvent); | 95% |
-
-
4389-45-1
3-methylantranilic acid

-
-
147695-56-5
4-bromophenyl isoselenocyanate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 40℃; for 4.5h; | 95% |
-
-
4389-45-1
3-methylantranilic acid

| Conditions | Yield |
|---|---|
| With bromine; acetic acid In dichloromethane at 20℃; for 1h; | 95% |
-
-
4389-45-1
3-methylantranilic acid

-
-
108-24-7
acetic anhydride

-
-
67081-69-0
2-acetylamino-3-methylbenzoic acid

| Conditions | Yield |
|---|---|
| for 2h; Reflux; | 94% |
| for 2h; Heating; | 87% |
| for 1h; Acetylation; Heating; | 85% |
| at 60℃; Reflux; |
-
-
4389-45-1
3-methylantranilic acid

-
-
66176-17-8
6-methylisatoic anhydride

| Conditions | Yield |
|---|---|
| With bis(trichloromethyl) carbonate; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; dichloromethane at 20℃; for 16.5h; | 94% |
| Multi-step reaction with 2 steps 1: triethylamine; dmap / acetonitrile / 2 h / 20 °C 2: 2-chloro-1-methyl-pyridinium iodide / acetonitrile / 0.17 h / 20 °C View Scheme |
-
-
4389-45-1
3-methylantranilic acid

-
-
3473-63-0
formamidine acetic acid

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
19181-54-5
8-methylquinazolin-4(3H)-one

| Conditions | Yield |
|---|---|
| at 180℃; for 3h; | 94% |

-
-
6089-09-4
4-pentynoic acid

-
-
4389-45-1
3-methylantranilic acid

-
-
1227624-59-0
3a,9-dimethyl-3,3a-dihydro-1H-benzo[d]pyrrolo[2,1-b][1,3]-oxazine-1,5(2H)-dione

| Conditions | Yield |
|---|---|
| With Echavarren's catalyst In 1,1-dichloroethane at 120℃; for 12h; Sealed vial; | 93% |
3-Methylanthranilic acid Chemical Properties
IUPAC Name: 2-Amino-3-methylbenzoic acid
Synonyms of 3-Methylanthranilic acid (CAS NO.4389-45-1): 2-Amino-3-methylbenzoic acid ; 3-Methyl-2-aminobenzoic acid ; Benzoic acid, 2-amino-3-methyl- (9CI) ; m-Toluic acid, 2-amino- (8CI)
CAS NO: 4389-45-1
Molecular Formula: C8H9NO2
Molecular Weight: 151.16
Molecular Structure: 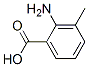
H bond acceptors: 3
H bond donors: 3
Freely Rotating Bonds: 2
Polar Surface Area: 29.54 Å2
Index of Refraction: 1.618
Molar Refractivity: 42.24 cm3
Molar Volume: 120.5 cm3
Surface Tension: 58.2 dyne/cm
Density: 1.254 g/cm3
Flash Point: 144.4 °C
Enthalpy of Vaporization: 58.74 kJ/mol
Boiling Point: 315.1 °C at 760 mmHg
Vapour Pressure: 0.000188 mmHg at 25°C
Melting Point: 174-177 °C(lit.)
Appearance: pink to grey-brown crystalline powder
Product Categories of 3-Methylanthranilic acid (CAS NO.4389-45-1): CARBOXYLICACID;FINE Chemical & INTERMEDIATES;Amines;blocks;Carboxes;Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;Carboxylic Acids;Phenyls & Phenyl-Het;Benzoic acid;Organic acids;Carboxylic Acids;Phenyls & Phenyl-Het
3-Methylanthranilic acid Uses
3-Methylanthranilic acid (CAS NO.4389-45-1) is used as intermediates in organic synthesis.
3-Methylanthranilic acid Safety Profile
Safety Information about 3-Methylanthranilic acid (CAS NO.4389-45-1):
Hazard Codes:  Xn,Xi
Xn,Xi
Risk Statements: 22-36/37/38
R22: Harmful if swallowed.
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 24/25-36-26
S24/25: Avoid contact with skin and eyes.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36: Wear suitable protective clothing.
WGK Germany: 3
F: 10
Hazard Note: Irritant
HazardClass: IRRITANT
3-Methylanthranilic acid Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media: In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage: Store in a cool, dry place. Keep container closed when not in use.
Related Products
- 3-Methylanthranilic acid
- 4389-50-8
- 4390-04-9
- 4390-94-7
- 439108-20-0
- 439117-40-5
- 439118-88-4
- 439-14-5
- 439213-22-6
- 4392-24-9
- 4392-52-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View