-
Name
4-Hexanolide
- EINECS 211-778-8
- CAS No. 695-06-7
- Article Data70
- CAS DataBase
- Density 1.003 g/cm3
- Solubility 77g/L at 20℃
- Melting Point -18 °C
- Formula C6H10O2
- Boiling Point 214.904 °C at 760 mmHg
- Molecular Weight 114.144
- Flash Point 79.338 °C
- Transport Information
- Appearance colourless to pale yellow liquid with a herbaceous, sweet odour
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Caproicacid, g-hydroxy-, lactone (4CI);Hexanoic acid, 4-hydroxy-, g-lactone (7CI);4-Ethyl-4-butanolide;4-Hydroxyhexanoic acid lactone;5-Ethyldihydro-2(3H)-furanone;5-Ethyltetrahydro-2-furanone;g-Ethyl-g-butyrolactone;
- PSA 26.30000
- LogP 1.10200
Synthetic route

| Conditions | Yield |
|---|---|
| With dodecacarbonyltetrarhodium(0); triphenylphosphine; isopropyl alcohol at 220℃; for 1h; | 91% |

-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With nickel; isopropyl alcohol for 0.25h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With calcium(II) bis(trifluoromethanesulfonyl)imide; tert-butylammonium hexafluorophosphate(V) at 80℃; for 2h; Sealed tube; | 88% |
| With chloro-trimethyl-silane; water; sodium iodide In hexane for 44h; Ambient temperature; | 66% |
-
-
823-22-3, 26991-67-3
5-hexanolide

-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With W(OTf)6 at 180℃; for 10h; Schlenk technique; Ionic liquid; Inert atmosphere; Green chemistry; | 86% |
| With hydrogen iodide at 125℃; for 4h; |
-
-
128756-18-3
4-Methoxymethoxy-hexanoic acid trimethylhydrazide

-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With perchloric acid In dichloromethane for 8h; Ambient temperature; | 85% |

| Conditions | Yield |
|---|---|
| With W(OTf)6 at 180℃; for 10h; Catalytic behavior; Reagent/catalyst; Temperature; Time; Schlenk technique; Ionic liquid; Inert atmosphere; Green chemistry; | 85% |
| With silica-alumina In water at 374.84℃; under 750.075 Torr; Kinetics; Flow conditions; | |
| With W(OTf)6 at 150℃; for 10h; Reagent/catalyst; Temperature; Green chemistry; | 99 %Chromat. |
-
-
51827-43-1
γ-Hydroxycapronitril

-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With Rhodococcus rhodochrous IFO 15564 at 30℃; phosphate buffer, pH 6; | 79% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In nitromethane for 3h; Heating; | 77% |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| In benzene alkalical hydrolysis; | 71% |
-
-
19781-77-2
hept-6-en-3-ol

-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With cethyltrimethylammonium permanganate In chloroform at 25℃; for 4h; | 69% |
| With (bipyH2)-CrOCl5 In dichloromethane Heating; | 40% |

| Conditions | Yield |
|---|---|
| With hydrogen iodide at 125℃; for 4h; | A 67% B 7% C 14% |


| Conditions | Yield |
|---|---|
| With hydrogen iodide at 125℃; for 4h; | 67% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 130℃; Yields of byproduct given; | A 62% B n/a |

| Conditions | Yield |
|---|---|
| With sodium persulfate In water at 85 - 90℃; for 5h; | A 60% B 22% |

| Conditions | Yield |
|---|---|
| With W(OTf)6 at 180℃; for 10h; Schlenk technique; Ionic liquid; Inert atmosphere; Green chemistry; | 57% |
| With aluminium(III) triflate at 200℃; for 10h; Reagent/catalyst; Temperature; Green chemistry; | 96 %Chromat. |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; water; sodium iodide In hexane for 18h; Heating; | 54% |

| Conditions | Yield |
|---|---|
| With chromium(V) | A 40% B 50% |
| With potassium permanganate; water; copper(II) sulfate In dichloromethane 1.) reflux, 5 min, 2.) RT, 4 h; Yield given. Yields of byproduct given; |
-
-
628-62-6
heptanamide

-
A
-
695-06-7
hexan-4-olide

-
B
-
142-62-1
hexanoic acid

-
C
-
823-22-3, 26991-67-3
5-hexanolide

| Conditions | Yield |
|---|---|
| With sodium persulfate; copper dichloride at 85 - 90℃; for 5h; | A 38% B 42% C 18% |


| Conditions | Yield |
|---|---|
| Stage #1: methylmagnesium bromide With copper(l) iodide In tetrahydrofuran; diethyl ether at 0℃; for 0.5h; Inert atmosphere; Stage #2: methyl 3-(oxiran-2-yl)propanoate In tetrahydrofuran; diethyl ether at -30℃; for 2h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; diethyl ether; water | 42% |
-
-
628-02-4
Caproamide

-
A
-
695-06-7
hexan-4-olide

-
B
-
142-62-1
hexanoic acid

-
C
-
823-22-3, 26991-67-3
5-hexanolide

| Conditions | Yield |
|---|---|
| With sodium persulfate; sodium chloride In water at 85 - 90℃; for 10h; | A 40% B 41% C 19% |
| With sodium persulfate; sodium chloride In water at 85 - 90℃; for 10h; | A 40 % Chromat. B 41 % Chromat. C 19% |
| With sodium persulfate; sodium chloride In water at 85 - 90℃; for 10h; | A 40 % Chromat. B 41 % Chromat. C 19 % Chromat. |


| Conditions | Yield |
|---|---|
| Stage #1: methyllithium With copper(I) cyanide In tetrahydrofuran; diethyl ether at 0℃; for 0.5h; Inert atmosphere; Stage #2: methyl 3-(oxiran-2-yl)propanoate In tetrahydrofuran; diethyl ether at -30℃; for 2h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; diethyl ether; water | 40% |

| Conditions | Yield |
|---|---|
| With 1-Cyanonaphthalene; biphenyl In water; acetonitrile for 13.3333h; | 36% |

| Conditions | Yield |
|---|---|
| Stage #1: methyllithium With copper(l) iodide In diethyl ether at 0℃; for 0.25h; Inert atmosphere; Stage #2: 5-bromomethyl-γ-butyrolactone In diethyl ether at -70 - 0℃; for 5.5h; Inert atmosphere; Stage #3: With ammonium chloride In diethyl ether; water | 34% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid; zinc at 85℃; | |
| With hydrogenchloride; tin(ll) chloride at 85℃; Reagens 4: Eisessig; | |
| With hydrogenchloride; tin at 85℃; Reagens 4: Eisessig; |
-
-
28274-28-4
2-ethyl-5-oxo-tetrahydro-furan-3-carboxylic acid

-
A
-
695-06-7
hexan-4-olide

-
B
-
4219-24-3
hex-3-enoic acid

| Conditions | Yield |
|---|---|
| bei der Destillation; |
-
-
127727-92-8
trans-3-hexenenitrile

-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
13419-69-7
(E)-2-Hexenoic acid

-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With PPA at 70℃; |

| Conditions | Yield |
|---|---|
| With sodium dichromate; dimethylsulfide borane complex; sulfuric acid 1.) Et2O, a) -78 to 25 deg C, b) 25 deg C, 12 h, 2.) reflux, 1 h; Yield given. Multistep reaction; |
-
-
13532-38-2, 87241-93-8, 97189-67-8
γ-Hydroxycaproic acid

-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 0℃; for 1h; Yield given; |
-
-
695-06-7
hexan-4-olide

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

| Conditions | Yield |
|---|---|
| With C12H36MgN2Si4 In neat (no solvent) at 20℃; for 0.25h; Catalytic behavior; Reagent/catalyst; Solvent; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; W(OTf)6; hydrogen at 135℃; under 760.051 Torr; for 12h; | 97% |
| With palladium on activated carbon; W(OTf)6; hydrogen In neat (no solvent) at 135℃; under 760.051 Torr; for 12h; | 94% |

| Conditions | Yield |
|---|---|
| With hexafluoroantimonic acid at 0℃; for 1h; | 96% |

| Conditions | Yield |
|---|---|
| With sodium methylate In toluene at 50℃; for 70h; Inert atmosphere; | 94% |
-
-
695-06-7
hexan-4-olide

-
-
16432-53-4
hexane-1,4-diol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 0 - 20℃; for 16h; Inert atmosphere; | 92% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; | 77% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 2h; Ambient temperature; Yield given; |
-
-
695-06-7
hexan-4-olide

| Conditions | Yield |
|---|---|
| With Lawessons reagent; Hexamethyldisiloxane at 120℃; for 0.025h; microwave irradiation; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: Benzyl propargyl ether With n-butyllithium In tetrahydrofuran at -78 - -30℃; Stage #2: hexan-4-olide With boron trifluoride diethyl etherate In tetrahydrofuran at -78 - 20℃; for 1h; | 92% |
-
-
695-06-7
hexan-4-olide

-
-
30414-44-9
5-ethyltetrahydrofuran-2-ol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In hexane; dichloromethane at -78℃; for 1h; | 91% |
| With diisobutylaluminium hydride In tetrahydrofuran at -78℃; | 85% |
| Stage #1: hexan-4-olide With diisobutylaluminium hydride In toluene at -63 - -61℃; for 1.5h; Inert atmosphere; Stage #2: With methanol In diethyl ether; toluene at 20℃; | 52% |
| Stage #1: hexan-4-olide With diisobutylaluminium hydride In toluene at -63 - -61℃; for 1.5h; Inert atmosphere; Stage #2: With methanol In diethyl ether; toluene at 20℃; | |
| Stage #1: hexan-4-olide With diisobutylaluminium hydride In hexane; dichloromethane at -75 - -70℃; for 4.5h; Stage #2: With water; ammonium chloride In hexane; dichloromethane Cooling with ice; |
-
-
695-06-7
hexan-4-olide

-
-
75-77-4
chloro-trimethyl-silane

-
-
34939-91-8, 58648-56-9
dimethyl lithiomethylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: hexan-4-olide; dimethyl lithiomethylphosphonate Stage #2: With lithium diisopropyl amide Stage #3: chloro-trimethyl-silane | 90% |


| Conditions | Yield |
|---|---|
| at 130℃; for 2h; | 89% |
-
-
695-06-7
hexan-4-olide

-
-
40726-47-4
4-hydroxyhexanamide

| Conditions | Yield |
|---|---|
| With ammonia at 20℃; for 240h; | 87% |
| With ethanol; ammonia at 100℃; | |
| With ammonia; water durch Schuetteln; |

| Conditions | Yield |
|---|---|
| Stage #1: hexan-4-olide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 1.25h; Stage #2: diphenyl diselenide With N,N,N,N,N,N-hexamethylphosphoric triamide In tetrahydrofuran at -78 - -40℃; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl propionate With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; Stage #2: hexan-4-olide In tetrahydrofuran; hexane at -78℃; for 2h; Stage #3: tert-butyldimethylsilyl chloride With tert-butoxide In tetrahydrofuran at 20℃; | 86% |

-
-
695-06-7
hexan-4-olide

-
-
540-88-5
acetic acid tert-butyl ester

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: acetic acid tert-butyl ester With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; Stage #2: hexan-4-olide In tetrahydrofuran; hexane at -78℃; Stage #3: tert-butyldimethylsilyl chloride With tert-butoxide In tetrahydrofuran at 20℃; | 85% |

-
-
695-06-7
hexan-4-olide

-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
1360562-58-8
4-hydroxy-N-methoxy-N-methylhexanamide

| Conditions | Yield |
|---|---|
| With isopropylmagnesium chloride In tetrahydrofuran at -20 - -15℃; for 0.333333h; Inert atmosphere; | 85% |

| Conditions | Yield |
|---|---|
| With 1,2-bis-(diphenylphosphino)ethane; methyl iodide; palladium(II) acetylacetonate at 80 - 90℃; for 12h; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: hexan-4-olide With sodium hydroxide In ethanol Stage #2: With sodium hypochlorite In aq. phosphate buffer at 20℃; for 20h; | 81% |
| With tetraethylammonium bromide In N,N-dimethyl-formamide at 20℃; | 72% |
| With sodium hydroxide; bromine; magnesium sulfate | |
| Multi-step reaction with 2 steps 1: NaOH / ethanol 2: NaOCl / aq. phosphate buffer / 20 h / 20 °C / pH 6.6 View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: O-benzylhydoxylamine hydrochloride With trimethylaluminum In tetrahydrofuran; toluene at 0 - 20℃; for 0.833333h; Stage #2: hexan-4-olide In tetrahydrofuran; toluene at 0℃; for 1.5h; | 80% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80 - 85℃; for 16h; Heating; Large scale; | 72.8% |
| In ethyl acetate |

| Conditions | Yield |
|---|---|
| With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; lithium diisopropyl amide In tetrahydrofuran at -78℃; | 68% |
| Stage #1: hexan-4-olide With N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium; diisopropylamine In tetrahydrofuran at -78℃; for 2h; Inert atmosphere; Stage #2: ethyl iodide In tetrahydrofuran at -78℃; for 1h; Inert atmosphere; |
4-Hexanolide Consensus Reports
4-Hexanolide Specification
The 4-Hexanolide, with the CAS registry number 695-06-7, is also known as 5-Ethyldihydro-2(3H)-furanone. It belongs to the product categories of Heterocycles; Lactone Flavors. Its EINECS registry number is 211-778-8. This chemical's molecular formula is C6H10O2 and molecular weight is 114.14. What's more, its IUPAC name is called 5-Ethyloxolan-2-one. It should be stored in a cool, dry and well-ventilated place.
Physical properties about 4-Hexanolide are: (1)ACD/LogP: 0.413; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.41; (4)ACD/LogD (pH 7.4): 0.41; (5)ACD/BCF (pH 5.5): 1.21; (6)ACD/BCF (pH 7.4): 1.21; (7)ACD/KOC (pH 5.5): 39.98; (8)ACD/KOC (pH 7.4): 39.98; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.432; (14)Molar Refractivity: 29.509 cm3; (15)Molar Volume: 113.829 cm3; (16)Polarizability: 11.698×10-24cm3; (17)Surface Tension: 29.002 dyne/cm; (18)Density: 1.003 g/cm3; (19)Flash Point: 79.338 °C; (20)Enthalpy of Vaporization: 45.124 kJ/mol; (21)Boiling Point: 214.904 °C at 760 mmHg; (22)Vapour Pressure: 0.152 mmHg at 25 °C.
Preparation of 4-Hexanolide: this chemical can be prepared by hept-6-en-3-ol. This reaction needs reagent cetyltrimethylammonium permanganate and solvent CHCl3 at temperature of 25 °C. The reaction time is 4 hours. The yield is 69 %.

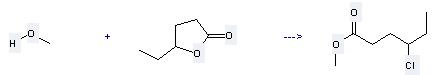
Uses of 4-Hexanolide: (1) it is used as flavor; (2) it is used to produce other chemicals. For example, it can react with methanol to get 4-chloro-hexanoic acid methyl ester. The reaction occurs with reagent HCl and solvent methanol at temperature of 0 °C. The yield is 48 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause inflammation to the skin or other mucous membranes. And it is irritating to eyes, respiratory system and skin. Therefore, you should wear suitable protective clothing. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C1OC(CC)CC1
(2) InChI: InChI=1S/C6H10O2/c1-2-5-3-4-6(7)8-5/h5H,2-4H2,1H3
(3) InChIKey: JBFHTYHTHYHCDJ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 794, 1979. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 794, 1979. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View