-
Name
2-Methyl-4-methoxybenzenamine
- EINECS 203-036-7
- CAS No. 102-50-1
- Article Data32
- CAS DataBase
- Density 1.065 g/cm3
- Solubility insoluble,
- Melting Point 13-14 °C(lit.)
- Formula C8H11NO
- Boiling Point 249.4 °C at 760 mmHg
- Molecular Weight 137.181
- Flash Point 113.5 °C
- Transport Information
- Appearance brick red crystals or liquid
- Safety 26-36/37/39-53-45
- Risk Codes 36/37/38-40-45
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn,
Xn, T
T
- Synonyms p-Anisidine,2-methyl- (7CI,8CI);2-Amino-5-methoxytoluene;2-Methyl-4-(methyloxy)aniline;2-Methyl-4-anisidine;2-Methyl-4-methoxyaniline;2-Methyl-4-methoxybenzenamine;2-Methyl-p-anisidine;4-Methoxy-2-methylaniline;4-Methoxy-2-methylbenzenamine;4-Methoxy-o-toluidine;NSC 66563;[2-Methyl-4-(methyloxy)phenyl]amine;
- PSA 35.25000
- LogP 2.16700
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In isopropyl alcohol under 2327.2 Torr; | 98.5% |
| With sodium formate In ethanol at 85℃; for 1h; | 87% |
| With tetrahydroxydiboron; water at 80℃; for 8h; | 80% |
-
-
208399-66-0
4-methoxy-2-methylphenyl boronic acid

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With sodium hydroxide; hydroxylamine-O-sulfonic acid In acetonitrile at 20℃; for 16h; | 89% |
-
-
27060-75-9
4-bromo-3-methylanisole

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With ammonium sulfate; (R)-(-)-1-[(SP)-2-(dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine; bis(tri-ortho-tolylphosphine)palladium(0); sodium t-butanolate In 1,4-dioxane at 100℃; for 6h; Inert atmosphere; Glovebox; | 80% |
| With ammonium hydroxide; copper(l) iodide; potassium carbonate; 4R-4-hydroxyproline In dimethyl sulfoxide at 70℃; for 36h; | 77% |
-
-
154876-10-5
1-(4-methoxy-2-methylphenyl)-1,2-hydrazinedicarboxylic acid bis(2,2,2-trichloroethyl) ester

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With acetic acid; zinc for 0.75h; Ambient temperature; | 76% |
-
-
13334-71-9
1-chloro-4-methoxy-2-methylbenzene

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; potassium phosphate; copper(l) iodide; N1,N2-bis(2,4,6-trimethoxyphenyl)oxalamide In water; dimethyl sulfoxide at 120℃; for 48h; Inert atmosphere; | 64% |
| With bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; N-[2-(di(1-adamantyl)phosphino)phenyl]morpholine; ammonia; sodium t-butanolate In 1,4-dioxane at 65℃; for 24h; Inert atmosphere; chemoselective reaction; | 52% |
-
-
31601-41-9
N-(4-methoxy-2-methylphenyl)acetamide

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 25℃; durch wochenlange Einw.; |
-
-
100-17-4
para-methoxynitrobenzene

-
-
676-58-4
methylmagnesium chloride

-
A
-
5961-59-1
N-methyl-N-(4-methoxyphenyl)amine

-
B
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; palladium on activated charcoal 1) THF, 20 min, -30 deg C, 2) 20 deg C, 2 h; Yield given. Multistep reaction. Yields of byproduct given; |


-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
7647-01-0
hydrogenchloride

-
-
75112-71-9
1-(4-methoxy-2-methyl-phenyl)-ethanone oxime

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 92 percent / ZnCl2 / diethyl ether; CH2Cl2 / 0.5 h / 25 °C 2: 76 percent / Zn, glacial acetic acid / 0.75 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate; xylene 2: tin (II)-chloride; hydrochloric acid View Scheme | |
| Multi-step reaction with 2 steps 1: alkaline solution 2: tin; hydrochloric acid; alcohol View Scheme | |
| Multi-step reaction with 2 steps 1: diethyl ether 2: tin; hydrochloric acid View Scheme | |
| Multi-step reaction with 2 steps 1: (methylation) 2: N2H4*H2O / Raney-Ni View Scheme |
-
-
39495-15-3
4-acetamido-3-methylphenol

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: alkali 2: hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: HNO3, AcOH 2: (methylation) 3: N2H4*H2O / Raney-Ni View Scheme |

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With glycine ethyl ester hydrochloride; triethylamine In methanol; water at 40℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: [bis(acetoxy)iodo]benzene / 0 - 20 °C / Inert atmosphere 2: triethylamine; glycine ethyl ester hydrochloride / methanol; water / 40 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: [bis(acetoxy)iodo]benzene / 0.53 h / 0 - 20 °C / Inert atmosphere 1.2: 1 h / 0 - 20 °C / Inert atmosphere 2.1: [bis(acetoxy)iodo]benzene / 0 - 20 °C / Inert atmosphere 3.1: triethylamine; glycine ethyl ester hydrochloride / methanol; water / 40 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: [bis(acetoxy)iodo]benzene / 0 - 20 °C / Inert atmosphere 2: triethylamine; glycine ethyl ester hydrochloride / methanol; water / 40 °C / Inert atmosphere View Scheme |
-
-
57197-11-2
4,4-dimethoxy-2-methylcyclohexane-2,5-dien-1-one

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With glycine ethyl ester hydrochloride; triethylamine In methanol; water at 40℃; Inert atmosphere; | 93 %Spectr. |
-
-
13091-23-1
4-chloro-3-nitropyridine

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
1513682-06-8
N-(4-methoxy-2-methylphenyl)-3-nitropyridin-4-amine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 20℃; for 16h; | 100% |
| With triethylamine In ethanol at 20℃; for 16h; | 100% |
| With triethylamine In ethanol at 20℃; for 16h; | 100% |

| Conditions | Yield |
|---|---|
| In various solvent(s) at 110℃; pH=3; | 99% |
-
-
1885-14-9
phenyl chloroformate

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
1378364-99-8
phenyl N-(4-methoxy-2-methylphenyl)carbamate

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 0 - 20℃; | 99% |
| With pyridine In tetrahydrofuran at 0 - 20℃; | 81.1% |
-
-
4530-20-5
BOC-glycine

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
1448525-54-9
[(4-methoxy-2-methylphenylcarbamoyl)methyl]carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 5h; Inert atmosphere; | 99% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 5h; |
-
-
108-24-7
acetic anhydride

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
31601-41-9
N-(4-methoxy-2-methylphenyl)acetamide

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 20℃; Inert atmosphere; | 98% |
| In dichloromethane at 25℃; for 3h; Large scale; | 91.8% |
| In dichloromethane at 20℃; | 82% |
-
-
87-13-8
diethyl 2-ethoxymethylenemalonate

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
384820-97-7
diethyl 2-((4-methoxy-2-methylphenylamino)methylene)malonate

| Conditions | Yield |
|---|---|
| at 120℃; for 1h; | 98% |
| In toluene for 5h; Reflux; | 14.4 g |
-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-4-methoxyaniline With tetrafluoroboric acid at 0℃; Stage #2: With sodium nitrite at 0 - 20℃; for 3h; Further stages.; | 98% |
| With tetrafluoroboric acid; sodium nitrite at 0℃; | 96% |
| Stage #1: 2-methyl-4-methoxyaniline With boron trifluoride diethyl etherate In tetrahydrofuran for 0.0833333h; Cooling with ice; Stage #2: With tert.-butylnitrite In tetrahydrofuran at 0℃; | 90% |
| Stage #1: 2-methyl-4-methoxyaniline With tetrafluoroboric acid In water at 20℃; Stage #2: With sodium nitrite In water at 10℃; Cooling with ice; | 78% |
| Stage #1: 2-methyl-4-methoxyaniline With tetrafluoroboric acid In water at 20℃; for 0.166667h; Stage #2: With sodium nitrite In water at 0 - 20℃; for 1h; |

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); sodium tert-pentoxide; XPhos In 1,4-dioxane at 110℃; for 12h; Buchwald-Hartwig Coupling; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0℃; for 0.5h; | 97% |
| With sodium carbonate In dichloromethane; water at 80℃; for 2h; | 93% |
| With triethylamine In ethyl acetate |
-
-
7338-94-5
1,3-diphenyl-2-propynone

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
1198463-75-0
(Z)-3-(4-methoxy-2-methylphenylamino)-1,3-diphenylprop-2-en-1-one

| Conditions | Yield |
|---|---|
| In methanol at 80℃; for 1h; Sealed tube; | 96% |
| In ethanol Michael Addition; Reflux; | 96% |
-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
109463-66-3
N-TMS-4-Methoxy-2-methylaniline

| Conditions | Yield |
|---|---|
| With cyclohexane-1,2-epoxide; lithium iodide; chloro-trimethyl-silane In neat (no solvent) for 1h; Heating; | 95% |

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With acetic acid In acetonitrile at 160℃; for 0.166667h; microwave irradiation; | 95% |
-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With acetic acid In acetonitrile at 160℃; for 0.166667h; microwave irradiation; | 95% |
-
-
108-90-7
chlorobenzene

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
41317-15-1
4-phenylamino-3-methyl-1-methoxybenzene

| Conditions | Yield |
|---|---|
| With potassium phosphate; DavePhos; tris(dibenzylideneacetone)dipalladium (0) In toluene at 110℃; for 8h; Buchwald-Hartwig amination; | 95% |
| With potassium phosphate; DavePhos; tris-(dibenzylideneacetone)dipalladium(0) In toluene Heating; | 95% |
-
-
108-86-1
bromobenzene

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
41317-15-1
4-phenylamino-3-methyl-1-methoxybenzene

| Conditions | Yield |
|---|---|
| With caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; tris(dibenzylideneacetone)dipalladium (0) In toluene at 110℃; Buchwald-Hartwig amination; | 95% |
| With 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; tris-(dibenzylideneacetone)dipalladium(0) In toluene Heating; | 95% |
| With potassium phosphate; copper(l) iodide; C18H15N3O2 at 25℃; for 24h; Reagent/catalyst; Temperature; Sealed tube; | 93% |
| With potassium phosphate; copper(l) iodide In diethylene glycol at 70℃; for 14h; Sealed tube; | 93% |
-
-
796048-82-3
5-(4-benzyloxy-benzenesulfonyl)-4-chloro-2-methyl-pyrimidine

-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 1h; Heating / reflux; | 95% |
-
-
95-46-5
2-methylphenyl bromide

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
100925-29-9
4-methoxy-2-methyl-N-(2-tolyl)aniline

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; sodium t-butanolate In toluene at 100℃; for 4h; | 95% |
-
-
102-50-1
2-methyl-4-methoxyaniline

-
A
-
41317-15-1
4-phenylamino-3-methyl-1-methoxybenzene

-
B
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| A n/a B 94% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol In acetonitrile at 80℃; for 12h; Sealed tube; Green chemistry; | 94% |
-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With acetic acid In acetonitrile at 160℃; for 0.166667h; microwave irradiation; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,2-trifluroethylamine hydrochloride With acetic acid; sodium nitrite In dichloromethane; water at 20℃; for 0.5h; Stage #2: 2-methyl-4-methoxyaniline With C50H23Cl8FeN4O10 In dichloromethane; water for 12.5h; | 93% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; meso-tetraphenylporphyrin iron(III) chloride; 1,1,1,3',3',3'-hexafluoro-propanol at 20℃; regioselective reaction; | 93% |
-
-
102-50-1
2-methyl-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With acetic acid In acetonitrile at 160℃; for 0.166667h; microwave irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; triethylamine; triphenylphosphine for 3h; Heating; | 92% |
| With triethylamine; triphenylphosphine In tetrachloromethane for 3h; Heating; | 92% |
| Stage #1: trifluoroacetic acid With tetrachloromethane; triethylamine; triphenylphosphine at 0℃; for 0.166667h; Inert atmosphere; Stage #2: 2-methyl-4-methoxyaniline at 0 - 85℃; Inert atmosphere; | 89% |
-
-
4635-59-0
4-Chlorobutanoyl chloride

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
727993-66-0
1-[2-methyl-4-(methyloxy)phenyl]-2-pyrrolidinone

| Conditions | Yield |
|---|---|
| Stage #1: 4-Chlorobutanoyl chloride; 2-methyl-4-methoxyaniline With triethylamine In tetrahydrofuran at 0 - 10℃; for 1.5h; Stage #2: With potassium tert-butylate In tetrahydrofuran at 0 - 10℃; for 2h; | 92% |
-
-
16714-25-3
2-azidobenzaldehyde

-
-
102-50-1
2-methyl-4-methoxyaniline

-
-
1393125-78-4
2-(4-methoxy-2-methylphenyl)-2H-indazole

| Conditions | Yield |
|---|---|
| at 100℃; under 10343.2 Torr; Microwave irradiation; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-4-methoxyaniline With tetrafluoroboric acid; tert.-butylnitrite In ethanol; water at 0 - 20℃; Stage #2: 2,3-diethynylquinoxaline With tetrabutylammonium tetrafluoroborate; trifluoroacetic acid In acetonitrile at 20℃; for 4.5h; Electrochemical reaction; | 92% |

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol In acetonitrile at 80℃; for 12h; Sealed tube; Green chemistry; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: sodium carbonate; chlorobenzene With copper In N,N-dimethyl-formamide; toluene for 0.25h; Reflux; Stage #2: 2-methyl-4-methoxyaniline In N,N-dimethyl-formamide; toluene under 760.051 Torr; for 10.6h; Reagent/catalyst; Solvent; Reflux; | 91.47% |

4-Methoxy-2-methylaniline Chemical Properties
MW: 137.18
EINECS: 203-036-7
2-METHYL-p-ANISIDINE(102-50-1),Its melting point is about 13-14 °C(lit.);boiling point is 248-249 °C(lit.);density is 1.065 g/mL at 25 °C(lit.) .Following is the molecular structure of 2-METHYL-p-ANISIDINE(102-50-1):
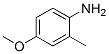
4-Methoxy-2-methylaniline Uses
4-Methoxy-2-methylaniline Toxicity Data With Reference
| 1. | otr-rat:emb 51,500 ng/plate | JJATDK JAT, Journal of Applied Toxicology. 1 (1981),190. |
RTECS :BZ6730000
4-Methoxy-2-methylaniline Consensus Reports
4-Methoxy-2-methylaniline Safety Profile
Suspected carcinogen with experimental carcinogenic and tumorigenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Other safty informations about 2-METHYL-p-ANISIDINE(102-50-1):
Hazard Codes: Xi,Xn,T (Irritant;Harmful;Toxic)
Risk Statements :
R36/37/38:Irritating to eyes, respiratory system and skin
R40:Limited evidence of a carcinogenic effect
R45 :May cause cancer
Safety Statements :
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection
S53:Avoid exposure - obtain special instruction before use
S45 :In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
RIDADR : 2810
WGK Germany : 3
Related Products
- 4-Methoxy-2-methylaniline
- 1025010-58-5
- 102503-23-1
- 102504-75-6
- 102506-12-7
- 10250-63-2
- 10250-64-3
- 102507-13-1
- 102507-85-7
- 102510-57-6
- 10251-17-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View