-
Name
4-Methylpyrazole
- EINECS 231-445-0
- CAS No. 7554-65-6
- Article Data19
- CAS DataBase
- Density 1.062 g/cm3
- Solubility soluble
- Melting Point 13°C
- Formula C4H6N2
- Boiling Point 243.6 °C at 760 mmHg
- Molecular Weight 82.105
- Flash Point 96.1 °C
- Transport Information UN 2810
- Appearance clear colourless to yellowish liquid after melting
- Safety 26-36-24/25
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Pyrazole,4-methyl- (6CI,7CI,8CI);4-Methyl-1H-pyrazole;Antizol;Fomepizole;Fomepizolum;
- PSA 28.68000
- LogP 0.71810
Synthetic route
-
-
10602-37-6
1,1,3,3-tetraethoxy-2-methyl-propane

-
-
7554-65-6
4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,3,3-tetraethoxy-2-methyl-propane With hydrazinium sulfate In water at 80℃; for 3h; Stage #2: With sodium hydroxide In water at 3 - 30℃; pH=4 - 6; Stage #3: With sodium hydrogencarbonate In water at 27 - 30℃; pH=7; | 84% |
| Stage #1: 1,1,3,3-tetraethoxy-2-methyl-propane With hydrazinium sulfate In water at 80℃; for 3h; Stage #2: With sodium hydroxide In water at 3 - 30℃; pH=4 - 6; Stage #3: With sodium hydrogencarbonate In water at 27℃; pH=7; | 84% |

-
-
7554-65-6
4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 1h; Heating; | 68% |
-
-
99968-85-1
4-methyl-1H-pyrazole-3,5-dicarboxylic acid

-
-
7554-65-6
4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| bei der trocknen Destillation des Silbersalzes; |
-
-
82231-51-4
4-methyl-1H-pyrazole-3-carboxylic acid

-
-
7554-65-6
4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| With soda lime Beim Destillieren; |

| Conditions | Yield |
|---|---|
| With calcium carbonate In 1,4-dioxane at 85 - 110℃; Yield given; | |
| With calcium carbonate In 1,4-dioxane at 85 - 110℃; Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With calcium carbonate In 1,4-dioxane at 85 - 110℃; Yield given. Yields of byproduct given; |

-
-
7554-65-6
4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| With ethanol; hydrazine hydrate |
-
-
82231-51-4
4-methyl-1H-pyrazole-3-carboxylic acid

-
-
7554-65-6
4-methyl-1H-pyrazole
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
30989-81-2
3-amino-2-methyl acrolein

-
-
7554-65-6
4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| With tetrabutyl-ammonium chloride In water at 20 - 80℃; for 4h; Reagent/catalyst; Temperature; | 82 g |

| Conditions | Yield |
|---|---|
| 100% | |
| 92.1% | |
| phosphoric acid | 68% |
| With sodium hydroxide; dimethyl sulfate | |
| With sulfuric acid In methanol |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
121669-69-0
4-methylpyrazole-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dmap In acetonitrile for 1h; | 100% |
| With dmap In dichloromethane at 20℃; for 1h; | 93% |
| With dmap In acetonitrile at 20℃; | 84% |
-
-
7554-65-6
4-methyl-1H-pyrazole


| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 18h; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With NaOH In dichloromethane byproducts: NaCl; stirring of equimolar amts. of CuCl2*2H2O, 4-methylpyrazole, NaOH and N(C4H9)4Cl in CH2Cl2 for 12 h at ambient temp.; filtration, treatment with Et2O, filtration, washing with Et2O, recrystn. from CH2Cl2/Et2O; elem. anal.; | 99% |


| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; JoSPOPhos SL-J688-2; pyridinium p-toluenesulfonate In toluene at 80℃; for 16h; Inert atmosphere; Schlenk technique; Sealed tube; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With hydroxybenzaldoxime; copper(I) oxide In acetonitrile at 82℃; for 24h; | 98% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
1174132-49-0
4-methyl-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazole

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In dichloromethane; toluene at 55℃; for 16h; | 98% |
| With pyridinium p-toluenesulfonate In dichloromethane at 55℃; for 12h; | 61% |
| With trifluoroacetic acid In toluene at 80℃; Large scale; |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
1448803-95-9
1,2,4,5-tetrafluoro-3-(1H-indol-1-yl)benzene

-
-
1610894-52-4
1-(2,3,5,6-tetrakis(4-methyl-1H-pyrazol-1-yl)phenyl)-1H-indole

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In N,N-dimethyl acetamide at 20℃; for 24h; | 98% |
-
-
7554-65-6
4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| Stage #1: (E)-2-(prop-1-en-1-yl)-1-tosylbenzimidazole With (R)-3,3'-bis(9-anthracenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In tetrahydrofuran at -40℃; for 0.166667h; Michael Addition; Inert atmosphere; Stage #2: 4-methyl-1H-pyrazole In tetrahydrofuran at -40℃; for 48h; Michael Addition; Inert atmosphere; enantioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate In acetonitrile at 80℃; for 5h; Schlenk technique; regioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 120℃; for 24h; Inert atmosphere; | 98% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
1448804-06-5
1-(2,3,5,6-tetrafluorophenyl)-1H-benzo[d]imidazole

-
-
1610894-50-2
1-(2,3,5,6-tetrakis(4-methyl-1H-pyrazol-1-yl)phenyl)-1H-benzo[d]imidazole

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In N,N-dimethyl acetamide at 20℃; for 24h; | 97% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
38858-90-1
4-methyl-3-nitro-1H-pyrazole

| Conditions | Yield |
|---|---|
| With silica-sulfuric acid impregnated with bismuth nitrate In tetrahydrofuran at 20℃; for 6h; Reagent/catalyst; Green chemistry; | 96% |
| With bismuth(III) nitrate In tetrahydrofuran at 20℃; for 3.5h; | 84% |
| With sulfuric acid; nitric acid at 30 - 105℃; for 2h; | 55% |
| With sulfuric acid; nitric acid at 100℃; for 2h; | 20 %Spectr. |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 45℃; for 10h; Inert atmosphere; Electrochemical reaction; Green chemistry; diastereoselective reaction; | 96% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
854044-35-2
1-(5-fluoro-2-nitrophenyl)piperidine

-
-
885704-59-6
1-[5-(4-methyl-pyrazol-1-yl)-2-nitro-phenyl]-piperidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dimethyl sulfoxide at 90℃; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: aniline With tert.-butylnitrite; acetic acid In methanol at 0℃; for 0.5h; Schlenk technique; Inert atmosphere; Stage #2: 4-methyl-1H-pyrazole With copper diacetate In methanol at 0 - 20℃; for 14h; Schlenk technique; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| In water Sealed tube; | 95% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
22931-71-1
ethyl 4-chloro-6-methoxyquinolin-3-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 100℃; for 2h; Sealed tube; Microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether; toluene at 0℃; for 1h; | 94% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
1035347-03-5
9-(2,3,5,6-tetrafluorophenyl)-9H-carbazole

-
-
1610894-53-5
9-(2,3,5,6-tetrakis(4-methyl-1H-pyrazol-1-yl)phenyl)-9H-carbazole

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In N,N-dimethyl acetamide at 20℃; for 24h; | 94% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
247043-64-7
C13H15NO2

| Conditions | Yield |
|---|---|
| With lithium chloride In acetonitrile for 9h; Schlenk technique; Reflux; | 93% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
19614-12-1
2-bromo-5-methoxybenzyl bromide

-
-
1252590-76-3
1-(2-bromo-5-methoxybenzyl)-4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-5-methoxybenzyl bromide With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 0.5h; Stage #2: 4-methyl-1H-pyrazole In tetrahydrofuran; mineral oil at 20℃; for 12h; | 92% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
66790-58-7
2-bromo-1-(bromomethyl)-3-methylbenzene

-
-
1252590-77-4
1-(2-bromo-3-methylbenzyl)-4-methyl-1H-pyrazole

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-1-(bromomethyl)-3-methylbenzene With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 0.5h; Stage #2: 4-methyl-1H-pyrazole In tetrahydrofuran; mineral oil at 20℃; for 12h; | 92% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
1262133-94-7
1-(2,3,5,6-tetrafluorophenyl)-1H-pyrazole

-
-
1610894-51-3
1,1',1'',1'''-(3-(1H-pyrazol-1-yl)benzene-1,2,4,5-tetrayl)tetrakis(4-methyl-1H-pyrazole)

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In N,N-dimethyl acetamide at 20℃; for 24h; | 92% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
586-78-7
para-nitrophenyl bromide

-
-
13808-73-6
4-methyl-1-(4-nitrophenyl)-1H-pyrazole

| Conditions | Yield |
|---|---|
| With C31H28NO6PPdS; caesium carbonate In water at 90℃; for 24h; Schlenk technique; Inert atmosphere; | 92% |
| With potassium tert-butylate In dimethylsulfoxide-d6 at 60℃; for 5h; | 80% |
-
-
7554-65-6
4-methyl-1H-pyrazole

-
-
13360-57-1
dimethylamino sulfonyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methyl-1H-pyrazole With sodium hydride In melt; mineral oil for 0.0833333h; Inert atmosphere; Stage #2: trifluoromethyl trifluorovinyl ether In melt; mineral oil | 92% |
4-Methylpyrazole Specification
The CAS registry number of 4-Methylpyrazole is 7554-65-6. Its EINECS registry number is 231-445-0. The IUPAC name is 4-methyl-1H-pyrazole. In addition, the molecular formula is C4H6N2 and the molecular weight is 82.10. It is also called pyrazole, 4-methyl-. What's more, it is a kind of clear colourless to yellowish liquid after melting and belongs to the classes of Imidazoles, Pyrroles, Pyrazoles, Pyrrolidines. And it should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 0.78; (2)ACD/LogD (pH 5.5): 0.77; (3)ACD/LogD (pH 7.4): 0.78; (4)ACD/BCF (pH 5.5): 2.27; (5)ACD/BCF (pH 7.4): 2.29; (6)ACD/KOC (pH 5.5): 62.29; (7)ACD/KOC (pH 7.4): 63.07; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)Polar Surface Area: 17.82 Å2; (11)Index of Refraction: 1.523; (12)Molar Refractivity: 23.6 cm3; (13)Molar Volume: 77.2 cm3; (14)Polarizability: 9.35 ×10-24cm3; (15)Surface Tension: 43.7 dyne/cm; (16)Density: 1.062 g/cm3; (17)Flash Point: 96.1 °C; (18)Enthalpy of Vaporization: 46.11 kJ/mol; (19)Boiling Point: 243.6 °C at 760 mmHg; (20)Vapour Pressure: 0.0497 mmHg at 25°C.
Preparation of 4-Methylpyrazole: it can be prepared by 4-methyl-5-trimethylsilanyl-1H-pyrazole. This reaction will need reagent aq. KOH and solvent ethanol. The reaction time is 1 hour by heating. The yield is about 68%.
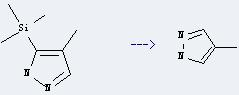
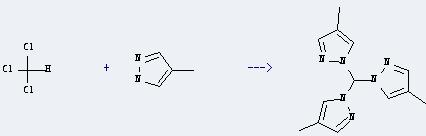
Uses of 4-Methylpyrazole: it can be used as an antidote in confirmed or suspected methanol or ethylene glycol. It may be used alone or in combination with hemodialysis. In addition, it can react with trichloromethane to get tris(4-methylpyrazol-1-yl)methane. This reaction will need reagents K2CO3 and tetrabutylammonium bromide. The reaction time is 10 hours by heating. The yield is about 40%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful if swallowed and irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: n1cc(cn1)C
(2)InChI: InChI=1/C4H6N2/c1-4-2-5-6-3-4/h2-3H,1H3,(H,5,6)
(3)InChIKey: RIKMMFOAQPJVMX-UHFFFAOYAT
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 310mg/kg (310mg/kg) | Experientia. Vol. 28, Pg. 1198, 1972. | |
| mouse | LD50 | oral | 640mg/kg (640mg/kg) | Experientia. Vol. 28, Pg. 1198, 1972. | |
| rat | LD50 | intravenous | 310mg/kg (310mg/kg) | Experientia. Vol. 28, Pg. 1198, 1972. | |
| rat | LD50 | oral | 534mg/kg (534mg/kg) | Experientia. Vol. 28, Pg. 1198, 1972. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View