-
Name
4-N-PROPYLBENZALDEHYDE
- EINECS 249-221-6
- CAS No. 28785-06-0
- Article Data23
- CAS DataBase
- Density 0.985 g/cm3
- Solubility
- Melting Point 19 °C
- Formula C10H12O
- Boiling Point 239.907 °C at 760 mmHg
- Molecular Weight 148.205
- Flash Point 96.544 °C
- Transport Information
- Appearance
- Safety 26-36
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Benzaldehyde,p-propyl- (8CI);4-(n-Propyl)benzaldehyde;p-Propylbenzaldehyde;
- PSA 17.07000
- LogP 2.45160
Synthetic route

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate In water at 30℃; for 0.0833333h; | 100% |
-
-
89557-35-7
4-n-propylbenzaldehyde diethyl acetal

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| In(OSO2CF3)3 In acetone at 20℃; for 0.5h; | 94% |
| With acetic acid In water for 17h; Heating; | 91% |
| With trifluoroacetic acid In dichloromethane at 25℃; for 20h; | 83% |
| With hydrogenchloride In tetrahydrofuran for 6.5h; Heating; |
-
-
13402-36-3
1-propyl-1,3,5,7-cyclooctatetraene

-
A
-
28785-06-0
4-n-propylbenzaldehyde

-
B
-
59059-44-8
2-propylbenzaldehyde

-
C
-
103528-31-0
3-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With oxygen; copper(l) chloride; palladium dichloride In tetrahydrofuran; water at 60℃; for 6h; | A 5% B 15% C 80% |


| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 80℃; Heating; | 70% |
| In trifluoroacetic acid at 80℃; |
-
-
3166-97-0
1-(Chloromethyl)-4-propylbenzene

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With hexamethylenetetramine In acetic acid | |
| With ethanol; hexamethylenetetramine |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; hydrogen; palladium on activated charcoal 1.) tetrachloroethane, rt, 30 min; 2.) 100 deg C, normal pressure or 50 deg C, 30 atm; AcONa can be added; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In chloroform at -20℃; for 0.75h; |
-
-
52710-27-7
4-propylbenzoyl chloride

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With tributyl-amine; hydrogen; palladium on activated charcoal In benzene for 6h; Ambient temperature; |
-
-
103-65-1
Propylbenzene

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. HCl, H3PO4 / acetic acid 2: urotropine / acetic acid View Scheme | |
| Multi-step reaction with 2 steps 1: AlCl3 / trichloroethene / 0.5 h / Ambient temperature 2: H2; tributylamine / 10percent Pd/C / benzene / 6 h / Ambient temperature View Scheme |
-
-
2438-05-3
4-n-propylbenzoic acid

-
-
28785-06-0
4-n-propylbenzaldehyde
-
-
1122-91-4
4-bromo-benzaldehyde

-
-
77047-87-1
isopropyl zinc bromide

-
A
-
28785-06-0
4-n-propylbenzaldehyde

-
B
-
122-03-2
(4-isopropylbenzaldehyde)

| Conditions | Yield |
|---|---|
| With [1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride In tetrahydrofuran; toluene at 0 - 20℃; for 3h; Negishi coupling reaction; Inert atmosphere; |

-
-
1254940-88-9
4-propylbenzaldehyde dimethyl acetal

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With water | 14.6 %Chromat. |

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 20℃; for 1h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; aluminum (III) chloride In hexane at -25 - 13℃; under 21446.5 - 24032.3 Torr; for 2.08333h; Catalytic behavior; Pressure; Temperature; Solvent; Reagent/catalyst; Gatterman-Koch Carbonylation; Autoclave; Sealed tube; | 96.4 %Spectr. |
-
-
1074-55-1
p-n-propyltoluene

-
A
-
5337-93-9
4'-methylpropiophenone

-
B
-
28785-06-0
4-n-propylbenzaldehyde

-
C
-
208453-24-1
ethyl 4-formylphenyl ketone

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; oxygen; cobalt(II) diacetate tetrahydrate at 20℃; under 760.051 Torr; |

-
-
118-96-7
2,4,6-Trinitrotoluene

-
A
-
67-47-0
5-hydroxymethyl-2-furfuraldehyde

-
B
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With culture of Trichoderma viride |
-
-
2579-20-6
cis, trans-1,3-dimethylaminocyclohexane

-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
1217526-85-6
C28H38N2

| Conditions | Yield |
|---|---|
| In methanol at 20℃; Molecular sieve; | 100% |
| In methanol at 20℃; Molecular sieve; |

| Conditions | Yield |
|---|---|
| With poly(4-vinylpyridine)/AlPO4-11 In water at 20℃; Knoevenagel condensation; | 98% |
| With porous hierarchical MgO/Mg(OH)2 In water at 60℃; for 0.0833333h; Knoevenagel Condensation; | 94% |
-
-
667-27-6
Ethyl bromodifluoroacetate

-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
1359989-83-5
ethyl 2,2-difluoro-3-hydroxy-3-(4-propylphenyl)propanoate

| Conditions | Yield |
|---|---|
| Stage #1: Ethyl bromodifluoroacetate With zinc In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Stage #2: 4-n-propylbenzaldehyde In tetrahydrofuran for 4h; Inert atmosphere; Reflux; | 97.8% |
-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
1263037-12-2
ethyl 3-(4-propylphenyl)propionate

| Conditions | Yield |
|---|---|
| Stage #1: diethoxyphosphoryl-acetic acid ethyl ester; 4-n-propylbenzaldehyde With sodium ethanolate In ethanol; toluene at 0 - 25℃; for 4h; Stage #2: With palladium on activated charcoal; hydrogen In isopropyl alcohol at 20℃; | 95.5% |
-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With perchloric acid In ethanol at 20℃; for 0.5h; | 94% |
-
-
141-82-2
malonic acid

-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
62718-61-0
(E)-3-(4-n-propylphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| Stage #1: malonic acid; 4-n-propylbenzaldehyde With piperidine; pyridine Knoevenagel-Doebner reaction; Reflux; Stage #2: With hydrogenchloride In water Cooling with ice; optical yield given as %de; | 92% |
| With piperidine In pyridine at 120℃; for 4h; | |
| With piperidine In pyridine | |
| In pyridine at 80℃; for 2h; |

| Conditions | Yield |
|---|---|
| With beta zeolite modified with chlorosulphonic acid (28 wtpercent) at 20℃; Neat (no solvent); | 86% |

| Conditions | Yield |
|---|---|
| With ammonium acetate In neat (no solvent) at 60℃; for 0.5h; Green chemistry; | 86% |

-
-
3612-20-2
1-phenylmethyl-4-piperidone

-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
1550981-14-0
1-benzyl-3,5-bis(4-propylbenzylidene)piperidin-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol; water at 0℃; for 6h; Claisen-Schmidt Condensation; Reflux; | 83% |
-
-
88-15-3
2-Acetylthiophene

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With sodiumsulfide nonahydrate In methanol at 20℃; | 83% |
-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid for 24h; Knoevenagel Condensation; Reflux; | 82% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In water for 0.2h; Knoevenagel condensation; Reflux; | 81% |

-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
82657-70-3
4-n-propylbenzyl alcohol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran at 0℃; for 2h; | 80% |
| With sodium hydroxide; sodium tetrahydroborate In methanol; water for 0.583333h; Ambient temperature; | |
| With sodium tetrahydroborate; water In acetonitrile |
-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

| Conditions | Yield |
|---|---|
| In methanol for 0.25h; | 80% |
-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
1398498-90-2
2-{2-[(methylamino)methyl]-1H-benzimidazol-1-yl}acetohydrazide

-
-
1398499-13-2
N'-[(E)-(4-propylphenyl)methylidene]-2-{2-[(methylamino)methyl]-1H-benzimidazol-1-yl}acetohydrazide

| Conditions | Yield |
|---|---|
| With acetic acid In methanol Reflux; | 80% |
-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
36062-19-8
1,3-diaminoguanidine hydrochloride

| Conditions | Yield |
|---|---|
| In ethanol at 100℃; for 0.166667h; Microwave irradiation; | 77% |
-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium methylate; acetic acid for 24h; Reflux; | 77% |
-
-
1192-62-7
1-(2-furyl)-1-ethanone

-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With sodiumsulfide nonahydrate In methanol at 20℃; | 76% |
-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: (E)-(4-(3-oxo-3-phenylprop-1-en-1-yl) benzyl)triphenylphosphonium bromide With sodium hydroxide In dichloromethane at 0℃; for 0.166667h; Wittig Olefination; Stage #2: 4-n-propylbenzaldehyde In dichloromethane Wittig Olefination; | 75% |
-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
2615-25-0
trans-1,4-cyclohexyldiamine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; Molecular sieve; | 73% |
-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
99-93-4
4-Hydroxyacetophenone

| Conditions | Yield |
|---|---|
| Stage #1: 4-n-propylbenzaldehyde; 4-Hydroxyacetophenone With sodium hydroxide In methanol at 20℃; for 0.333333h; Claisen-Schmidt Condensation; Stage #2: In methanol at 20℃; Claisen-Schmidt Condensation; | 73% |
-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
78-94-4
methyl vinyl ketone

-
-
1033280-91-9
1-[4-(propyl)phenyl]-pentane-1,4-dione

| Conditions | Yield |
|---|---|
| With 3-ethyl-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide; triethylamine at 70℃; under 3102.97 Torr; for 0.25h; Stetter reaction; Microwave irradiation; | 72% |
| With 3-ethyl-5-(2-hydroxyethyl)-4-methyl-1,3-thiazolium bromide; triethylamine at 70℃; under 3102.97 Torr; for 0.25h; Stetter reaction; Microwave irradiation; | 63% |
-
-
28785-06-0
4-n-propylbenzaldehyde

| Conditions | Yield |
|---|---|
| With piperidine In ethanol Knoevenagel Condensation; Reflux; | 71% |
-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
1128-28-5
4-aminothymol

| Conditions | Yield |
|---|---|
| In methanol at 30 - 35℃; Inert atmosphere; | 68% |
-
-
1021268-16-5
1-(5-bromo-2-chloropyrimidine-4-yl)hydrazine

-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
1443042-23-6
2-(4-propylbenzylidene)-1-(5-bromo-2-chloropyrimidin-4-yl)hydrazine

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Reflux; | 68% |
-
-
28785-06-0
4-n-propylbenzaldehyde

-
-
6073-14-9
5-(tert-butyl)-4-hydroxy-2-methylaniline

-
-
1421830-44-5
C21H27NO

| Conditions | Yield |
|---|---|
| In methanol at 30 - 35℃; Inert atmosphere; | 65% |
4-Propylbenzaldehyde Consensus Reports
Reported in EPA TSCA Inventory.
4-Propylbenzaldehyde Specification
The 4-Propylbenzaldehyde, with the CAS registry number 28785-06-0, is also known as 4-(n-Propyl)benzaldehyde. It belongs to the product categories of Aldehyde; Aromatic Aldehydes & Derivatives (substituted); Aldehydes; C10 to C21; Carbonyl Compounds. Its EINECS number is 249-221-6. This chemical's molecular formula is C10H12O and molecular weight is 148.20. What's more, its systematic name is 4-Propylbenzaldehyde. This chemcial should be sealed and stored in a cool and dry place.
Physical properties of 4-Propylbenzaldehyde are: (1)ACD/LogP: 2.918; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.92; (4)ACD/LogD (pH 7.4): 2.92; (5)ACD/BCF (pH 5.5): 97.19; (6)ACD/BCF (pH 7.4): 97.19; (7)ACD/KOC (pH 5.5): 921.19; (8)ACD/KOC (pH 7.4): 921.19; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.54; (14)Molar Refractivity: 47.188 cm3; (15)Molar Volume: 150.423 cm3; (16)Polarizability: 18.707×10-24cm3; (17)Surface Tension: 36.1 dyne/cm; (18)Density: 0.985 g/cm3; (19)Flash Point: 96.544 °C; (20)Enthalpy of Vaporization: 47.681 kJ/mol; (21)Boiling Point: 239.907 °C at 760 mmHg; (22)Vapour Pressure: 0.04 mmHg at 25°C.
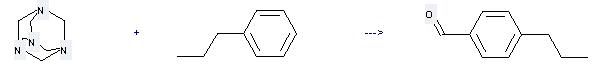
Preparation: this chemical can be prepared by 1,3,5,7-tetraaza-adamantane and propylbenzene by heating. This reaction will need reagent CF3COOH. The yield is about 70%.
Uses of 4-Propylbenzaldehyde: it can be used to produce 3-(4-propyl-phenyl)-propionic acid. It will need reagent (Et3N)2·(HCOOH)5. The yield is about 63%.
.jpeg)
When you are using this chemical, please be cautious about it as the following:
This chemcial is harmful if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)SMILES: O=Cc1ccc(cc1)CCC
(2)Std. InChI: InChI=1S/C10H12O/c1-2-3-9-4-6-10(8-11)7-5-9/h4-8H,2-3H2,1H3
(3)Std. InChIKey: MAUCRURSQMOFGV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 1600mg/kg (1600mg/kg) | Journal of Pharmaceutical Sciences. Vol. 63, Pg. 1068, 1974. |
Related Products
- 4-Propylbenzaldehyde
- 28787-22-6
- 28787-36-2
- 28788-62-7
- 28788-68-3
- 28789-35-7
- 28791-26-6
- 2879-15-4
- 287917-96-8
- 287917-97-9
- 2879-20-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View