-
Name
5-(2-Bromoethyl)-2,3-dihydrobenzofuran
- EINECS 603-191-4
- CAS No. 127264-14-6
- Article Data19
- CAS DataBase
- Density 1.459 g/cm3
- Solubility
- Melting Point 65-67 °C
- Formula C10H11BrO
- Boiling Point 287.7 °C 760 mmHg
- Molecular Weight 227.101
- Flash Point 113.8°C
- Transport Information
- Appearance off-white powder
- Safety
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,3-dihydro-5-(2-bromoethyl)benzofuran;
- PSA 9.23000
- LogP 2.55890
Synthetic route
-
-
87776-76-9
5-(2-hydroxyethyl)-2,3-dihydrobenzofuran

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In dichloromethane at 20℃; for 63h; | 84% |
| With phosphorus tribromide In chloroform Reflux; | 75.9% |
| 73% |
-
-
151427-19-9
2-bromo-1-(2,3-dihydrobenzofuran-5-yl)ethan-1-one

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-1-(2,3-dihydrobenzofuran-5-yl)ethan-1-one With sodium tetrahydroborate In tetrahydrofuran at 5 - 10℃; for 1h; Inert atmosphere; Stage #2: With aluminum (III) chloride In tetrahydrofuran at 45 - 50℃; for 4h; Inert atmosphere; | 74% |
-
-
943034-50-2
2-(2,3-dihydrobenzofuran-5-yl)ethylchloride

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| With sodium bromide In acetone for 48h; Heating / reflux; |
-
-
90843-31-5
1-(2,3-dihydrobenzofuran-5-yl)ethanone

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: toluene-4-sulfonic acid / methanol / 0.5 h / 0 - 5 °C 1.2: 6 h / 0 - 5 °C 2.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 5 - 10 °C / Inert atmosphere 2.2: 4 h / 45 - 50 °C / Inert atmosphere View Scheme |
-
-
496-16-2
2,3-Dihydrobenzofuran

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: aluminum (III) chloride / dichloromethane / 0.5 h / 0 - 5 °C / Inert atmosphere 1.2: 0.5 h / 0 - 5 °C / Inert atmosphere 2.1: toluene-4-sulfonic acid / methanol / 0.5 h / 0 - 5 °C 2.2: 6 h / 0 - 5 °C 3.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 5 - 10 °C / Inert atmosphere 3.2: 4 h / 45 - 50 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: aluminum (III) chloride / dichloromethane / 1 h / 0 - 10 °C 1.2: 10 - 20 °C 2.1: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 0 - 10 °C / Reflux 3.1: phosphorus tribromide / chloroform / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1.1: aluminum (III) chloride / dichloromethane / 1 h / 0 - 10 °C 1.2: 10 - 20 °C 2.1: water; sodium hydroxide / 20 °C 3.1: hydrazine hydrate; sodium hydroxide / 90 - 100 °C 4.1: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 0 - 10 °C / Reflux 5.1: phosphorus tribromide / chloroform / Reflux View Scheme |
-
-
64089-34-5
2-chloro-1-(2,3-dihydrobenzofuran-5-yl)ethanone

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate; sulfuric acid / tetrahydrofuran / 10 h / -5 - 0 °C 2: sodium bromide / acetone / 48 h / Heating / reflux View Scheme |
-
-
79002-49-6
ethyl 2( 2,3-dihydro-1-benzofuran-5-yl)oxoacetate

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: water; sodium hydroxide / 20 °C 2: hydrazine hydrate; sodium hydroxide / 90 - 100 °C 3: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 0 - 10 °C / Reflux 4: phosphorus tribromide / chloroform / Reflux View Scheme |

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrazine hydrate; sodium hydroxide / 90 - 100 °C 2: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 0 - 10 °C / Reflux 3: phosphorus tribromide / chloroform / Reflux View Scheme |
-
-
69999-16-2
2,3-dihydrobenzofuran-5-acetic acid

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 0 - 10 °C / Reflux 2: phosphorus tribromide / chloroform / Reflux View Scheme |
-
-
156-38-7
4-hydroxyphenylacetate

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: potassium iodide; sodium hydroxide / dimethyl sulfoxide / 50 - 80 °C 2: toluene-4-sulfonic acid / Reflux; Large scale 3: polyphosphoric acid / toluene / 105 - 110 °C 4: sodium hydroxide; water / methanol / 25 - 30 °C 5: sodium hydroxide; hydrogen / methanol; water / 60 - 65 °C / 6080.41 - 7600.51 Torr 6: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 7: phosphorus tribromide / chloroform / Reflux View Scheme | |
| Multi-step reaction with 7 steps 1: potassium iodide; sodium hydroxide / dimethyl sulfoxide / 50 - 80 °C 2: toluene-4-sulfonic acid / Reflux; Large scale 3: polyphosphoric acid / toluene / 105 - 110 °C 4: hydrogen; acetic acid; palladium 10% on activated carbon / methanol / 65 - 70 °C / 3040.2 - 3800.26 Torr 5: sodium hydroxide; water / methanol / 25 - 60 °C 6: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 7: phosphorus tribromide / chloroform / Reflux View Scheme |

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: toluene-4-sulfonic acid / Reflux; Large scale 2: polyphosphoric acid / toluene / 105 - 110 °C 3: hydrogen; acetic acid; palladium 10% on activated carbon / methanol / 65 - 70 °C / 3040.2 - 3800.26 Torr 4: sodium hydroxide; water / methanol / 25 - 60 °C 5: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 6: phosphorus tribromide / chloroform / Reflux View Scheme | |
| Multi-step reaction with 6 steps 1: toluene-4-sulfonic acid / Reflux; Large scale 2: polyphosphoric acid / toluene / 105 - 110 °C 3: sodium hydroxide; water / methanol / 25 - 30 °C 4: sodium hydroxide; hydrogen / methanol; water / 60 - 65 °C / 6080.41 - 7600.51 Torr 5: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 6: phosphorus tribromide / chloroform / Reflux View Scheme |

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: polyphosphoric acid / toluene / 105 - 110 °C 2: hydrogen; acetic acid; palladium 10% on activated carbon / methanol / 65 - 70 °C / 3040.2 - 3800.26 Torr 3: sodium hydroxide; water / methanol / 25 - 60 °C 4: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 5: phosphorus tribromide / chloroform / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1: polyphosphoric acid / toluene / 105 - 110 °C 2: sodium hydroxide; water / methanol / 25 - 30 °C 3: sodium hydroxide; hydrogen / methanol; water / 60 - 65 °C / 6080.41 - 7600.51 Torr 4: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 5: phosphorus tribromide / chloroform / Reflux View Scheme |
-
-
121638-36-6
methyl 2-(benzofuran-5-yl)acetate

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hydroxide; water / methanol / 25 - 30 °C 2: sodium hydroxide; hydrogen / methanol; water / 60 - 65 °C / 6080.41 - 7600.51 Torr 3: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 4: phosphorus tribromide / chloroform / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1: hydrogen; acetic acid; palladium 10% on activated carbon / methanol / 65 - 70 °C / 3040.2 - 3800.26 Torr 2: sodium hydroxide; water / methanol / 25 - 60 °C 3: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 4: phosphorus tribromide / chloroform / Reflux View Scheme |
-
-
142935-60-2
benzofuran-5-yl-acetic acid

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide; hydrogen / methanol; water / 60 - 65 °C / 6080.41 - 7600.51 Torr 2: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 3: phosphorus tribromide / chloroform / Reflux View Scheme |
-
-
155852-41-8
methyl 2-(2,3-dihydrobenzofuran-5-yl)acetate

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide; water / methanol / 25 - 60 °C 2: sodium tetrahydroborate; boron trifluoride diethyl etherate / tetrahydrofuran / 4 h / 10 °C / Reflux 3: phosphorus tribromide / chloroform / Reflux View Scheme |
-
-
292638-84-7
styrene

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate In 1,4-dioxane at 105℃; for 36h; Inert atmosphere; | 96% |

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
108-86-1
bromobenzene

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; Bathocuproine; tetrabutylammomium bromide; zinc at 20℃; regioselective reaction; | 95% |
| With nickel(II) bromide dimethoxyethane; [Ir(dF(CF3)ppy)2(phen)]PF6; Bathocuproine; diisopropylamine; magnesium bromide In N,N-dimethyl-formamide at 20℃; for 24h; Inert atmosphere; Glovebox; Sealed tube; Irradiation; regioselective reaction; | 87% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
133099-07-7
(S)-darifenacin hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dihydro-5-(2-bromoethyl)benzofuran; (S)-2,2-diphenyl-2-(pyrrolidin-3-yl)-acetamide L-tartrate With potassium hydroxide; triethylmethylammonium chloride In water; toluene at 100℃; for 15.25h; Stage #2: With hydrogen bromide In water; toluene Product distribution / selectivity; | 93.2% |
| Stage #1: (S)-2,2-diphenyl-2-(pyrrolidin-3-yl)-acetamide L-tartrate With potassium hydroxide; triethylmethylammonium chloride In 2-methyltetrahydrofuran; water at 60 - 70℃; Stage #2: 2,3-dihydro-5-(2-bromoethyl)benzofuran In 2-methyltetrahydrofuran; water for 16h; Heating / reflux; Stage #3: With hydrogen bromide In 2-methyltetrahydrofuran; water at 0 - 20℃; for 2h; Product distribution / selectivity; | 90.67% |
| Stage #1: 2,3-dihydro-5-(2-bromoethyl)benzofuran; (S)-2,2-diphenyl-2-(pyrrolidin-3-yl)-acetamide L-tartrate With potassium hydroxide; triethylmethylammonium chloride In water; butanone at 75℃; for 6h; Stage #2: With hydrogen bromide In water; butanone at 0 - 5℃; for 2h; Product distribution / selectivity; | 84.92% |

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; Bathocuproine; tetrabutylammomium bromide; zinc at 20℃; regioselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; Bathocuproine; tetrabutylammomium bromide; zinc at 20℃; regioselective reaction; | 90% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dihydro-5-(2-bromoethyl)benzofuran; 3-(S)-(+)-(1-cyano-1,1-diphenylmethyl)pyrrolidine hydrobromide With potassium carbonate In water at 75℃; for 4h; Stage #2: With hydrogen bromide In water | 89.93% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

| Conditions | Yield |
|---|---|
| Stage #1: 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane With bis(cyclopentadienyl)titanium dichloride; lithium methanolate In tert-butyl methyl ether for 0.5h; Stage #2: 2,3-dihydro-5-(2-bromoethyl)benzofuran In tert-butyl methyl ether at 60℃; under 760.051 Torr; for 24h; Inert atmosphere; | 89% |
| Stage #1: 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane With bis(cyclopentadienyl)titanium dichloride; lithium methanolate In tert-butyl methyl ether for 0.5h; Inert atmosphere; Glovebox; Stage #2: 2,3-dihydro-5-(2-bromoethyl)benzofuran In tert-butyl methyl ether at 60℃; for 24h; Sealed tube; | 89% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
151588-78-2
(2-bromo-2-fluoroethyl)benzene

| Conditions | Yield |
|---|---|
| With nickel(II) bromide dimethoxyethane; tetra-(n-butyl)ammonium iodide; potassium carbonate; 2,6-bis(4,5-dihydro-1H-imidazol-2-yl)pyridine; 2-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)-5,5-dimethyl-1,3,2-dioxaborinane In 1-methyl-pyrrolidin-2-one at 60℃; for 24h; Inert atmosphere; Sealed tube; Glovebox; | 88% |

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; bis(1,5-cyclooctadiene)nickel (0); zinc In N,N-dimethyl acetamide at 80℃; for 12h; | 87% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
134002-25-8
2,2-diphenyl-2-[(3S)-pyrrolidin-3-yl]acetamide

-
-
133099-07-7
(S)-darifenacin hydrobromide

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dihydro-5-(2-bromoethyl)benzofuran; 2,2-diphenyl-2-[(3S)-pyrrolidin-3-yl]acetamide With potassium hydroxide; triethylmethylammonium chloride In water; butanone at 75℃; for 6h; Heating / reflux; Stage #2: With hydrogen bromide In water; butanone | 84.92% |
| Stage #1: 2,3-dihydro-5-(2-bromoethyl)benzofuran; 2,2-diphenyl-2-[(3S)-pyrrolidin-3-yl]acetamide With potassium carbonate In acetonitrile at 70 - 75℃; for 2h; Stage #2: With hydrogen bromide In water; acetone at 0 - 25℃; Product distribution / selectivity; | |
| With potassium carbonate In acetonitrile |
-
-
108-30-5
succinic acid anhydride

-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; bis(1,5-cyclooctadiene)nickel (0); zinc In N,N-dimethyl acetamide at 80℃; for 12h; | 82% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; | 75% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
2675-79-8
1-bromo-3,4,5-trimethoxybenzene

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; Bathocuproine; tetrabutylammomium bromide; zinc at 20℃; regioselective reaction; | A 75% B n/a |


| Conditions | Yield |
|---|---|
| With nickel(II) iodide; C17H20N2O2; lithium bromide; zinc In 1-methyl-pyrrolidin-2-one at 30℃; for 24h; regioselective reaction; | 75% |

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; Bathocuproine; tetrabutylammomium bromide; zinc at 20℃; regioselective reaction; | 73% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
49562-28-9
fenofibrate

| Conditions | Yield |
|---|---|
| With 2.9-dimethyl-1,10-phenanthroline; nickel(II) perchlorate hexahydrate; tetrabutylammomium bromide In N,N-dimethyl acetamide at 20℃; for 10h; Glovebox; Sealed tube; Electrochemical reaction; Inert atmosphere; regioselective reaction; | 72% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
200064-11-5
5-bromo-2-(4-morpholinyl)pyridine

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; Bathocuproine; tetrabutylammomium bromide; zinc at 20℃; regioselective reaction; | 69% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
1423014-09-8
ethyl 4-phenyl-1,2,5,6-tetrahydropyridine-3-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dihydro-5-(2-bromoethyl)benzofuran; ethyl 4-phenyl-1,2,5,6-tetrahydropyridine-3-carboxylate With potassium carbonate; potassium iodide In acetonitrile Reflux; Inert atmosphere; Stage #2: With hydrogenchloride In diethyl ether; water Inert atmosphere; | 52% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
719278-77-0
(3S)-pyrrolidin-3-yl-cyclopentyl(hydroxy)phenyl acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 8h; Heating / reflux; | 50% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 8h; Heating / reflux; | 46% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
910108-52-0
C19H22N2O

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Heating / reflux; | 45% |

| Conditions | Yield |
|---|---|
| With norborn-2-ene; palladium diacetate; potassium carbonate at 80℃; for 18h; Inert atmosphere; | 28% |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
134002-25-8
2,2-diphenyl-2-[(3S)-pyrrolidin-3-yl]acetamide

-
-
133099-04-4
darifenacin

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 2h; Heating / reflux; | 9% |
| With potassium phosphate In water; toluene at 20 - 90℃; for 3.5h; Product distribution / selectivity; | |
| With potassium carbonate In water; toluene at 20 - 90℃; for 3.5h; Product distribution / selectivity; | |
| With potassium phosphate In cyclohexane; water at 20 - 90℃; for 3.5h; Product distribution / selectivity; | |
| With potassium hydroxide In acetonitrile at 40 - 50℃; for 18h; Product distribution / selectivity; |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

-
-
133099-07-7
(S)-darifenacin hydrobromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium hydroxide / acetonitrile / 18 h / 40 - 50 °C 2: hydrogen bromide / water; acetone / 2.25 - 12 h / 0 - 30 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / acetonitrile 2: ammonia / methanol / 5 - 7 h / 28 - 45 °C 3: hydrogen bromide / water; acetone / 2.25 - 12 h / 0 - 30 °C View Scheme |
-
-
127264-14-6
2,3-dihydro-5-(2-bromoethyl)benzofuran

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Heating / reflux; |
5-(2-Bromoethyl)-2,3-dihydrobenzofuran Chemical Properties
IUPAC Name: 5-(2-Bromoethyl)-2,3-dihydro-1-benzofuran
Synonyms of 5-(2-Bromoethyl)-2,3-dihydrobenzofuram (CAS NO.127264-14-6): 2,3-Dihydro-5-(2-bromoethyl)benzofuran ; 5-(2-Bromo ethyl)-2,3-dihydro benzofuran
CAS NO: 127264-14-6
Molecular Formula: C10H11BrO
Molecular Weight: 227.10
Molecular Structure: 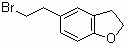
H bond acceptors: 1
H bond donors: 0
Freely Rotating Bonds: 2
Polar Surface Area: Å2
Index of Refraction: 1.592
Molar Refractivity: 52.7 cm3
Molar Volume: 155.5 cm3
Surface Tension: 46.7 dyne/cm
Density: 1.459 g/cm3
Flash Point: 113.8 °C
Enthalpy of Vaporization: 50.57 kJ/mol
Boiling Point: 287.7 °C at 760 mmHg
Vapour Pressure: 0.00424 mmHg at 25°C
Melting Point: 65-67°C
Appearance: Off-White Powder
SMILES: BrCCc1cc2CCOc2cc1
InChI: InChI=1/C10H11BrO/c11-5-3-8-1-2-10-9(7-8)4-6-12-10/h1-2,7H,3-6H2
InChIKey: JRKZQRRYNCMSCB-UHFFFAOYAA
Std. InChI: InChI=1S/C10H11BrO/c11-5-3-8-1-2-10-9(7-8)4-6-12-10/h1-2,7H,3-6H2
Std. InChIKey: JRKZQRRYNCMSCB-UHFFFAOYSA-N
Product Categories of 5-(2-Bromoethyl)-2,3-dihydrobenzofuram (CAS NO.127264-14-6): Pharmaceutical material and intermeidates;Fluorobenzene;APIs Intermediate;INTERMEDIATESOFDARIFENACIN;Furan&Benzofuran;API intermediates;Aromatics Compounds;Furans;Aromatics;Heterocycles;Benzodiozoles, Benzodioxines & Benzodioxepines;Methyl Halides;Benzofuran
5-(2-Bromoethyl)-2,3-dihydrobenzofuran Safety Profile
Hazard Codes:  Xi
Xi
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View