-
Name
5,7-DIBROMO-8-HYDROXYQUINOLINE
- EINECS 208-317-8
- CAS No. 521-74-4
- Article Data45
- CAS DataBase
- Density 2.052 g/cm3
- Solubility
- Melting Point 198-200 °C(lit.)
- Formula C9H5Br2NO
- Boiling Point 370.5 °C at 760 mmHg
- Molecular Weight 302.953
- Flash Point 177.9 °C
- Transport Information
- Appearance almost white powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 5,7-Dibromo-8-hydroxyquinoline;5,7-Dibromo-8-quinolinol;Brodiar;Broxyquinolin;Colepur;Colipar;Dibromoksin;Dibromoxine;Dibromoxyquinoline;Fenilor;Intensopan;NSC 1810;NSC 74937;Paramiba;
- PSA 33.12000
- LogP 3.46540
Synthetic route

| Conditions | Yield |
|---|---|
| With bromine; sodium hydrogencarbonate In methanol at 20℃; for 0.0833333h; | 99% |
| With bromine In methanol at 20℃; for 0.0833333h; | 97% |
| Stage #1: 8-quinolinol With bromine; sodium hydrogencarbonate In methanol at 20℃; for 0.0833333h; Stage #2: With sodium sulfite In methanol; water at 20℃; | 97% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium bromide at 25℃; bromination by the enzyme chloroperoxidase from Caldariomyces fumago in buffered solution of pH 2.7; | 79% |

| Conditions | Yield |
|---|---|
| With tribromo-isocyanuric acid In acetonitrile at 20℃; regioselective reaction; | A 72% B 11% |
| With bromine; acetic acid |

| Conditions | Yield |
|---|---|
| With bromine In chloroform at 20℃; for 48h; Darkness; | A 37% B 51% |
-
-
148-24-3
8-quinolinol

-
A
-
1198-14-7
5-bromo-8-hydroxyquinoline

-
B
-
521-74-4
broxyquinoline

-
C
-
13019-32-4
7-bromo-8-hydroxyquinoline

| Conditions | Yield |
|---|---|
| With hydrogen bromide; isopentyl nitrite In dichloromethane at 20℃; for 16h; | A 15% B 70 % Spectr. C 15% |

-
-
36107-02-5
5,7-dibromo-8-aminoquinoline

-
-
521-74-4
broxyquinoline

| Conditions | Yield |
|---|---|
| Diazotization.durch Kochen der Diazoniumsalz-Loesung mit Wasser; |
-
-
7647-01-0
hydrogenchloride

-
-
5852-78-8
8-hydroxy-quinoline-5-carboxylic acid

-
-
7726-95-6
bromine

-
-
521-74-4
broxyquinoline

-
-
7647-01-0
hydrogenchloride

-
-
101439-77-4
2-(8-hydroxy-quinoline-7-carbonyl)-benzoic acid

-
-
7726-95-6
bromine

-
-
521-74-4
broxyquinoline

-
-
2598-30-3
8-hydroxyquinoline-5-carbaldehyde

-
-
7726-95-6
bromine

-
-
64-19-7
acetic acid

-
-
521-74-4
broxyquinoline


-
-
521-74-4
broxyquinoline

| Conditions | Yield |
|---|---|
| With bromine; acetic acid | |
| With chloroform; bromine |

-
-
521-74-4
broxyquinoline

| Conditions | Yield |
|---|---|
| With bromine; acetic acid | |
| With chloroform; bromine |

-
-
521-74-4
broxyquinoline

| Conditions | Yield |
|---|---|
| With hydrogenchloride; bromine |

-
-
521-74-4
broxyquinoline

| Conditions | Yield |
|---|---|
| With bromine; acetic acid |

-
-
521-74-4
broxyquinoline

| Conditions | Yield |
|---|---|
| With bromine | |
| With phosphorus pentabromide |

-
-
521-74-4
broxyquinoline

| Conditions | Yield |
|---|---|
| beim Aufbewahren an der Luft, beim Kochen mit Wasser oder beim Erhitzen im Rohr auf 180grad; |
-
-
1555-94-8
8-ethoxyquinoline

-
-
7726-95-6
bromine

-
-
64-19-7
acetic acid

-
A
-
101422-06-4
8-ethoxy-5-bromo-quinoline

-
B
-
521-74-4
broxyquinoline



| Conditions | Yield |
|---|---|
| substance of Schmitt, Engelmann; |

-
-
56-23-5
tetrachloromethane

-
-
148-24-3
8-quinolinol

-
-
593-95-3
carbonyl dibromide

-
A
-
521-74-4
broxyquinoline


| Conditions | Yield |
|---|---|
| Reaktion des Hydrobromids; das entstehende rote Additionsprodukt uebergeht beim Aufbewahren unter Bromwasserstoff-Abspaltung; |

-
-
7732-18-5
water

-
-
7726-95-6
bromine

-
-
4053-45-6
5-chloromethyl-8-hydroxyquinoline hydrochloride

-
-
521-74-4
broxyquinoline

-
-
54250-31-6
tetrakis(5,7-dibromo-8-quinolinolato)thorium(IV) * 5,7-dibromo-8-quinolinol

-
A
-
521-74-4
broxyquinoline

-
B
-
107896-45-7
tetrakis(5,7-dibromo-8-quinolinolato)thorium(IV)

| Conditions | Yield |
|---|---|
| dissocn., 125-145°C; | |
| dissocn., 125-145°C; |

| Conditions | Yield |
|---|---|
| With bromine |
-
-
110-52-1
1,4-dibromo-butane

-
-
521-74-4
broxyquinoline

-
-
1026668-08-5
1,3-dibromo-7,8,9,10-tetrahydro-11-oxa-6a-azoniacycloocta[de]naphthalene bromide

| Conditions | Yield |
|---|---|
| With Amberlite IRA 402(OH) resin In water at 80℃; for 4h; Inert atmosphere; | 98% |
| With Amberlite IRA 402(OH) In water at 30 - 80℃; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With dmap In toluene at 130℃; for 1h; Microwave irradiation; Sealed tube; | 98% |
-
-
521-74-4
broxyquinoline

-
-
106-93-4
ethylene dibromide

-
-
1231156-93-6
7,9-dibromo-2,3-dihydro-1-oxa-3a-azonia-phenalene bromide

| Conditions | Yield |
|---|---|
| With Amberlite IRA 402(OH) resin In water at 80℃; for 3h; Inert atmosphere; | 97% |
| With Amberlite IRA 402(OH) In water at 30 - 80℃; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 1h; Inert atmosphere; | 96% |
-
-
3138-86-1
2,3-bis(bromomethyl)quinoxaline

-
-
521-74-4
broxyquinoline

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
1190620-41-7
4,6-bis(4-methoxyphenyl)-8,15-dihydro-1H-quinolino[8′,1′:2,3,4]-[1,4]oxazocino[6,7-b]quinoxalin-1-one

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In ethanol; water at 20℃; for 24h; Irradiation; Inert atmosphere; | 96% |


| Conditions | Yield |
|---|---|
| Stage #1: broxyquinoline With potassium carbonate In acetone for 0.5h; Reflux; Stage #2: dimethyl sulfate In acetone for 24h; Reflux; | 95.6% |

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; sodium hydroxide In water at 20℃; for 1h; | 95% |
-
-
521-74-4
broxyquinoline

-
-
1420334-85-5
and ethyl 2-(bromomethyl)benzo[h]quinoline-3-carboxylate

-
-
1420335-11-0
ethyl 2-((5,7-dibromoquinolin-8-yloxy)methyl)benzo[h]quinoline-3-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 4h; Williamson Ether Synthesis; Reflux; | 95% |
-
-
521-74-4
broxyquinoline

-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.5h; | 95% |
-
-
3138-86-1
2,3-bis(bromomethyl)quinoxaline

-
-
521-74-4
broxyquinoline

-
-
5720-05-8
4-methylphenylboronic acid

-
-
1190620-42-8
4,6-di-(p-tolyl)-8,15-dihydro-1H-quinolino[8′,1′:2,3,4][1,4]- oxazocino[6,7-b‑z]quinoxalin-1-one

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In ethanol; water at 20℃; for 24h; Irradiation; Inert atmosphere; | 95% |

-
-
110-52-1
1,4-dibromo-butane

-
-
521-74-4
broxyquinoline

-
-
1026668-09-6
1,3-dibromo-7,8,9,10-tetrahydro-11-oxa-6a-azoniacycloocta[de]naphthalen-6-one

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; sodium hydroxide In water at 20℃; for 1h; | 94% |
| With tert.-butylhydroperoxide; eosin y In ethanol; water at 20℃; for 24h; Inert atmosphere; Irradiation; |
-
-
521-74-4
broxyquinoline

-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane at 65℃; for 6h; Reflux; | 92.3% |
| With sodium methylate In methanol; chloroform at 20℃; for 1h; Inert atmosphere; Schlenk technique; | 76% |
-
-
521-74-4
broxyquinoline

-
-
111571-60-9
ethyl 2-bromomethyl-3-quinoline-3-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: broxyquinoline; ethyl 2-bromomethyl-3-quinoline-3-carboxylate With potassium carbonate In acetonitrile for 3h; Williamson Ether Synthesis; Reflux; Stage #2: With potassium hydroxide In ethanol; water for 2h; Williamson Ether Synthesis; Reflux; | 92% |
-
-
3138-86-1
2,3-bis(bromomethyl)quinoxaline

-
-
521-74-4
broxyquinoline

-
-
98-80-6
phenylboronic acid

-
-
1190620-40-6
4,6-diphenyl-8,15-dihydro-1H-quinolino[8′,1′:2,3,4][1,4]oxazocino[6,7-b]quinoxalin-1-one

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In ethanol; water at 20℃; for 24h; Reagent/catalyst; Solvent; Irradiation; Inert atmosphere; | 92% |

-
-
3138-86-1
2,3-bis(bromomethyl)quinoxaline

-
-
521-74-4
broxyquinoline

-
-
197958-29-5
pyridin-2-ylboronic acid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In ethanol; water at 20℃; for 24h; Irradiation; Inert atmosphere; | 92% |

-
-
521-74-4
broxyquinoline

-
-
111571-60-9
ethyl 2-bromomethyl-3-quinoline-3-carboxylate

-
-
1420334-93-5
ethyl 2-((5,7-dibromoquinolin-8-yloxy)methyl)quinoline-3-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 4h; Williamson Ether Synthesis; Reflux; | 91% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutylammomium bromide In tetrahydrofuran for 35h; | 90% |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; for 5h; | 89% |
| With sodium hydroxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; water at 40℃; | 78% |

| Conditions | Yield |
|---|---|
| With pyridine hydrochloride at 220℃; for 0.166667h; | 90% |
| With hydrogenchloride at 160 - 170℃; |
-
-
521-74-4
broxyquinoline

-
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
-
84165-49-1
5,7-dibromo-8-(2,4-dinitro-phenoxy)-quinoline

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane; water at 70℃; for 4h; | 90% |
-
-
521-74-4
broxyquinoline

-
-
638-38-0, 993-02-2, 2180-18-9, 15411-95-7, 22981-23-3, 27004-39-3
manganese(II) acetate

-
-
14495-08-0
5,7-dibromo-8-hydroxyquinolinato manganese(II)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water 1 M aq. Mn(OAc)2 (5 ml) added dropwise to soln. of 5,7-dibromo-8-hydroxyquinoline (10 mmol); refluxed (2 h); ppt. filtered; washed with EtOH; dried in air; | 90% |
-
-
3138-86-1
2,3-bis(bromomethyl)quinoxaline

-
-
521-74-4
broxyquinoline

-
-
1182708-31-1
5,7-dibromoquinolonoquinoxalino-oxazocine

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; sodium hydroxide In water at 20℃; for 1h; | 90% |
| With tetrabutylammomium bromide; sodium hydroxide In dichloromethane; water at 20℃; for 10h; | 88% |
-
-
521-74-4
broxyquinoline

-
-
3298-98-4
2,3-Bis(bromomethyl)-6,7-dimethylquinoxaline

-
-
1182708-27-5
C21H15Br2N3O2

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium hydroxide In dichloromethane; water at 20℃; for 12h; | 90% |
| With cetyltrimethylammonim bromide; sodium hydroxide In water at 20℃; for 1h; | 90% |
-
-
521-74-4
broxyquinoline

-
-
109-64-8
1,3-dibromo-propane

-
-
1026668-06-3
1,3-dibromo-8,9-dihydro-7H-10-oxa-6a-azacyclohepta[de]naphthalen-6-one

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; sodium hydroxide In water at 20℃; for 1h; | 90% |
| With tert.-butylhydroperoxide; eosin y In ethanol; water at 20℃; for 24h; Inert atmosphere; Irradiation; |

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; sodium hydroxide In water at 20℃; for 1h; | 90% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 13h; Inert atmosphere; | 90% |
-
-
13331-23-2
fur-2-ylboronic acid

-
-
3138-86-1
2,3-bis(bromomethyl)quinoxaline

-
-
521-74-4
broxyquinoline

-
-
1190620-45-1
4,6-di(furan-2-yl)-8,15-dihydro-1H-quinolino[8′,1′:2,3,4][1,4]oxazocino[6,7-b]-quinoxalin-1-one

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In ethanol; water at 20℃; for 24h; Irradiation; Inert atmosphere; | 90% |

-
-
3138-86-1
2,3-bis(bromomethyl)quinoxaline

-
-
521-74-4
broxyquinoline

-
-
32316-92-0
naphthalene-2-boronic acid

-
-
1190620-47-3
4,6-di(naphthalen-2-yl)-8,15-dihydro-1H-quinolino[8′,1′:2,3,4][1,4]oxazocino-[6,7-b]-quinoxalin-1-one

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In ethanol; water at 20℃; for 24h; Irradiation; Inert atmosphere; | 90% |

-
-
3138-86-1
2,3-bis(bromomethyl)quinoxaline

-
-
521-74-4
broxyquinoline

-
-
13922-41-3
1-Naphthylboronic acid

-
-
1190620-48-4
4,6-di(naphthalen-1-yl)-8,15-dihydro-1H-quinolino[8′,1′:2,3,4][1,4]oxazocino[6,7-b]-quinoxalin-1-one

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In ethanol; water at 20℃; for 24h; Irradiation; Inert atmosphere; | 90% |

-
-
521-74-4
broxyquinoline

| Conditions | Yield |
|---|---|
| With hydrogen; dihydrogen hexachloroplatinate In water; isopropyl alcohol at 90 - 95℃; under 15001.2 Torr; | 89% |
| With ammonia borane; tris(pentafluorophenyl)borate In toluene at 80℃; for 8h; Schlenk technique; Inert atmosphere; | 70% |
5,7-Dibromoquinolin-8-ol Chemical Properties
IUPAC Name: 5,7-Dibromoquinolin-8-ol
Synonyms: 5, 7-Dibromo-8-quinolinol ; 5, 7-Dibromoquinolin-8-ol ; 5,7-Dibromo-8-Hydroxyquinoline ; 5,7-dibromo-8-quinolinol ; 5,7-Dibromooxine ; 5,7-Dibromo-oxine
The Molecular Formula of 5, 7-Dibromo-8-quinolinol (521-74-4) :C9H5Br2NO
The Molecular Weight of 5, 7-Dibromo-8-quinolinol (521-74-4):302.9501 g/mol
The Molecular Structure of 5, 7-Dibromo-8-quinolinol (521-74-4):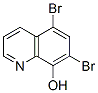
Index of Refraction: 1.738
Molar Refractivity: 59.44 cm3
Molar Volume: 147.5 cm3
Polarizability: 10-24 cm3
Surface Tension: 66.4 dyne/cm
Density: 2.052 g/ cm3
Flash Point: 177.9 °C
Enthalpy of Vaporization: 64.17 kJ/mol
Boiling Point: 370.5 °C at 760 mmHg
Vapour Pressure: 5.15E-06 mmHg at 25°C
5,7-Dibromoquinolin-8-ol Uses
5, 7-Dibromo-8-quinolinol (521-74-4) can be used as analytical reagent.
5,7-Dibromoquinolin-8-ol Toxicity Data With Reference
| 1. | orl-chd TDLo:1000 mg/kg/27D:CNS,PUL | LANCAO Lancet. 1 (1968),922. | ||
| 2. | orl-rat LDLo:10 g/kg | KSRNAM Kiso to Rinsho. Clinical Report. 4 (1970),27. | ||
| 3. | ipr-rat LD50:1140 mg/kg | KSRNAM Kiso to Rinsho. Clinical Report. 4 (1970),27. | ||
| 4. | orl-mus LD50:7420 mg/kg | KSRNAM Kiso to Rinsho. Clinical Report. 4 (1970),27. | ||
| 5. | ipr-mus LD50:325 mg/kg | KSRNAM Kiso to Rinsho. Clinical Report. 4 (1970),27. |
5,7-Dibromoquinolin-8-ol Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
5,7-Dibromoquinolin-8-ol Safety Profile
Poison by intraperitoneal route. Mildly toxic by ingestion. Human systemic effects by ingestion: muscle weakness, ataxia (loss of muscle coordination), and gastritis. When heated to decomposition it emits very toxic fumes of Br− and NOx.
Hazard Codes: Xi ![]()
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36: Wear suitable protective clothing
WGK Germany: 2
Related Products
- 5-0185 (Beilstein Handbook Reference) 6850-22-2
- 5,10,15,20-Tetra(4-methylphenyl)-21H,23H-porphine
- 5,10,15,20-Tetra(4-pyridyl)porphyrin
- 5,10,15,20-Tetrakis(2,4,6-trimethylphenyl)-21H,23H-porphine
- 5,10,15,20-Tetrakis(4-hydroxyphenyl)porphyrin
- 5,10,15,20-Tetrakis(4-methoxyphenyl)-21H,23H-porphine
- 5,10,15,20-Tetrakis(N-methyl-4-pyridyl)porphine tetratosylate
- 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin
- 5,10,15,20-Tetraphenyl-21H,23H-porphine cobalt(II)
- 5,10,15,20-Tetraphenyl-21H,23H-porphine iron(III) chloride
- 52175-10-7
- 52176-13-3
- 52178-50-4
- 52179-26-7
- 52179-72-3
- 52179-74-5
- 52182-15-7
- 52184-13-1
- 52184-14-2
- 52184-19-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View