-
Name
5-Amino-o-cresol
- EINECS 220-618-6
- CAS No. 2835-95-2
- Article Data16
- CAS DataBase
- Density 1.157 g/cm3
- Solubility insoluble in water
- Melting Point 160-162 °C(lit.)
- Formula C7H9NO
- Boiling Point 268.6 °C at 760 mmHg
- Molecular Weight 123.155
- Flash Point 116.3 °C
- Transport Information
- Appearance Beige crystals
- Safety 26-36-37/39
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms o-Cresol,5-amino- (6CI,7CI,8CI);1-Methyl-2-hydroxy-4-aminobenzene;2-Hydroxy-4-aminotoluene;2-Methyl-5-aminophenol;3-Amino-6-methylphenol;3-Hydroxy-4-methylaniline;3-Hydroxy-p-toluidine;4-Amino-2-hydroxytoluene;5-Amino-2-methylphenol;Phenol,5-amino-2-methyl-;6-Methyl-3-aminophenol;
- PSA 46.25000
- LogP 1.86400
Synthetic route

| Conditions | Yield |
|---|---|
| With copper; copper(l) chloride; sodium hydroxide In ethanol at 175℃; for 4h; Reagent/catalyst; Autoclave; Inert atmosphere; Industrial scale; | 85.36% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; antimony pentafluoride In hydrogen fluoride at -20℃; for 0.25h; Mechanism; Product distribution; | 71% |
| With dihydrogen peroxide; antimony pentafluoride In hydrogen fluoride at -20℃; for 0.25h; | 71% |
-
-
133914-73-5
N-(4-dimethylaminomethyl-3-hydroxyphenyl)acetamide

-
-
2835-95-2
amino-4 hydroxy-2 toluene

| Conditions | Yield |
|---|---|
| palladium on activated carbon In potassium hydroxide | 68% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin | |
| With palladium on activated charcoal; ethyl acetate Hydrogenation; | |
| With sodium hydroxide; sodium dithionite |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium nitrite; hydrochloric acid / anschliessend Verkochen 2: hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sulfuric acid; HNO3+H2SO4 / Verseifung der Acetylverbindung 2: concentrated sulfuric acid; water; NaNO2 / Diazotization 3: tin; hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sulfuric acid; nitric acid 2: concentrated sulfuric acid; water; NaNO2 / Diazotization 3: tin; hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid; water; NaNO2 / Diazotization 2: tin; hydrochloric acid View Scheme | |
| Multi-step reaction with 2 steps 1: (i) aq. NaNO2, H2SO4, (ii) aq. H2SO4 2: N2H4*H2O / Raney-Ni View Scheme |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
122-01-0
4-chloro-benzoyl chloride

-
-
929290-81-3
4-chloro-benzoic acid 5-(4-chloro-benzoylamino)-2-methyl-phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 16h; | 100% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
618-46-2
m-Chlorobenzoyl chloride

-
-
929291-99-6
3-chloro-benzoic acid 5-(3-chloro-benzoylamino)-2-methyl-phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 16h; | 100% |
| With triethylamine In tetrahydrofuran at 20℃; for 16h; |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
65055-17-6
3-chloro-4-fluorobenzoyl chloride

-
-
929291-96-3
3-Chloro-4-fluoro-benzoic acid 5-(3-chloro-4-fluoro-benzoylamino)-2-methyl-phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 16h; | 100% |
| With triethylamine In tetrahydrofuran at 20℃; for 16h; | |
| With triethylamine In tetrahydrofuran at 20℃; for 16h; |
-
-
590-28-3
potassium cyanate

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
16704-78-2
3-hydroxy-4-methyl-phenyl urea

| Conditions | Yield |
|---|---|
| With acetic acid In water at 35℃; for 24h; | 99% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
73387-74-3
4-chloro-7-methoxy-quinoline-3-carbonitrile

| Conditions | Yield |
|---|---|
| With pyridine hydrochloride In 2-ethoxy-ethanol for 1h; Heating / reflux; | 99% |
| With pyridine hydrochloride In 2-ethoxy-ethanol; water | 277 mg (99%) |
| With pyridine hydrochloride In 2-ethoxy-ethanol; water | 277 mg (99 %) |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
13744-00-8
2-(7-hydroxy-5,8-dimethyl-1,2,3,4-tetrahydronapthalen-2-yl)propanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-(7-hydroxy-5,8-dimethyl-1,2,3,4-tetrahydronapthalen-2-yl)propanoic acid With thionyl chloride In benzene Reflux; Stage #2: amino-4 hydroxy-2 toluene With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 6h; | 98% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
123-30-8
4-amino-phenol

-
-
30749-78-1
N-<-(4'-Hydroxy)-phenyl>-3-amino-6-methyl-benzochinon

| Conditions | Yield |
|---|---|
| With CotA-laccase from Bacillus subtilis; oxygen In aq. phosphate buffer; ethanol at 37℃; for 24h; pH=7; Green chemistry; Enzymatic reaction; | 98% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
388616-53-3
5-amino-4-iodo-2-methylphenol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium iodate; potassium iodide In water at 0 - 25℃; for 1h; | 97% |
| With [K(18-crown-6)]ICl2; calcium carbonate In acetonitrile at 50℃; for 4h; regioselective reaction; | 62% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
3282-30-2
pivaloyl chloride

-
-
1312305-91-1
N-(3-hydroxy-4-methylphenyl)-2,2-dimethylpropionamide

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; ethyl acetate at 20℃; for 1h; | 97% |
| With sodium hydrogencarbonate In water at 0 - 20℃; | 96% |
| Inert atmosphere; Schlenk technique; Alkaline conditions; |
-
-
6370-23-6
2,4-dimethylaniline-5-sulfonic acid

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
1357059-00-7
(E)-5-((2-amino-4-hydroxy-5-methylphenyl)diazenyl)-2,4-dimethylbenzenesulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,4-dimethylaniline-5-sulfonic acid With hydrogenchloride; sodium nitrite at 0℃; for 2h; Stage #2: amino-4 hydroxy-2 toluene With sodium hydroxide In water at 0℃; for 1h; pH=8 - 10; Inert atmosphere; | 95% |
| Stage #1: 2,4-dimethylaniline-5-sulfonic acid With hydrogenchloride; sodium nitrite In water at 0℃; for 2h; Stage #2: amino-4 hydroxy-2 toluene With sodium hydroxide In water at 0℃; pH=8; | 76.9% |

| Conditions | Yield |
|---|---|
| With 5-chloro-2-hydroxybenzoic acid; boric acid; palladium diacetate; 1,2-bis[di(t-butyl)phosphinomethyl]benzene In acetonitrile at 110℃; under 18100.7 Torr; for 48h; | 95% |

-
-
75-21-8
oxirane

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
55302-96-0
5-(2-hydroxyethylamino)-2-methylphenol

| Conditions | Yield |
|---|---|
| With NaY In diethylene glycol dimethyl ether at 150℃; for 3.5h; Reagent/catalyst; Solvent; Green chemistry; | 94.1% |
-
-
3993-78-0
2-amino-4-chloropyrimidine

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
933045-98-8
5-(2-aminopyrimidin-4-ylamino)-2-methylphenol

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-4-chloropyrimidine; amino-4 hydroxy-2 toluene With hydrogenchloride In ethanol; water at 90℃; for 18h; Stage #2: With potassium carbonate In ethanol; water; ethyl acetate Product distribution / selectivity; | 94% |
| With hydrogenchloride In ethanol; water at 80 - 90℃; Product distribution / selectivity; |
-
-
1092395-14-6
3-(1-cyanocyclopropyl)benzoyl chloride

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
1092395-08-8
3-(1-cyano-1-methylethyl)-N-(3-hydroxy-4-methylphenyl)benzamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran; water at 20℃; for 5h; | 94% |
-
-
556-96-7
5-bromo-1,3-xylene

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
1198117-30-4
5-(3,5-dimethylphenylamino)-2-methylphenol

| Conditions | Yield |
|---|---|
| With chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl][2-(2-aminoethyl)phenyl]palladium(ll); sodium t-butanolate In 1,4-dioxane at 90℃; for 1h; Inert atmosphere; | 94% |
-
-
1092395-13-5
3-(1-cyano-1-methylethyl)benzoyl chloride

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
1092395-08-8
3-(1-cyano-1-methylethyl)-N-(3-hydroxy-4-methylphenyl)benzamide

| Conditions | Yield |
|---|---|
| Stage #1: amino-4 hydroxy-2 toluene With sodium hydrogencarbonate In tetrahydrofuran; water at 20℃; Stage #2: 3-(1-cyano-1-methylethyl)benzoyl chloride In tetrahydrofuran; water at 20℃; Cooling with ice; | 94% |
-
-
2845-89-8
1-chloro-3-methoxy-benzene

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
1198117-41-7
3-[(3-methoxyphenyl)amino]-6-methylphenol

| Conditions | Yield |
|---|---|
| With chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl][2-(2-aminoethyl)phenyl]palladium(ll); sodium t-butanolate In 1,4-dioxane at 90℃; for 1.16667h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; palladium diacetate; toluene-4-sulfonic acid In tetrahydrofuran; acetonitrile at 120℃; under 23272.3 Torr; for 72h; regioselective reaction; | 92% |

-
-
88327-91-7
4-(pyrrolidin-1-ylsulfonyl)aniline

-
-
2835-95-2
amino-4 hydroxy-2 toluene

| Conditions | Yield |
|---|---|
| Stage #1: 4-(pyrrolidin-1-ylsulfonyl)aniline With hydrogenchloride; isopentyl nitrite In methanol; water; acetonitrile at 0℃; for 1h; Inert atmosphere; Stage #2: amino-4 hydroxy-2 toluene With potassium carbonate In methanol; water; acetonitrile at 0℃; for 1h; | 92% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
13669-62-0
4-chloro-6-methoxy-quinoline-3-carbonitrile

| Conditions | Yield |
|---|---|
| With pyridine hydrochloride In 2-ethoxy-ethanol for 1h; Heating / reflux; | 91% |
| With pyridine hydrochloride In 2-ethoxy-ethanol; water | 278.3 mg (91%) |
| With pyridine hydrochloride In 2-ethoxy-ethanol; water | 278.3 mg (91 %) |
-
-
2398-37-0
3-methoxyphenyl bromide

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
1198117-41-7
3-[(3-methoxyphenyl)amino]-6-methylphenol

| Conditions | Yield |
|---|---|
| With chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl][2-(2-aminoethyl)phenyl]palladium(ll); sodium t-butanolate In 1,4-dioxane at 90℃; for 1h; Inert atmosphere; | 91% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
98-88-4
benzoyl chloride

-
-
92199-49-0
N-(3-hydroxy-4-methylphenyl)benzamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; ethyl acetate at 0℃; for 2h; Inert atmosphere; Schlenk technique; | 91% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 0.5h; | 81% |
-
-
22065-57-2
ethyl 2-phenyldiazoacetate

-
-
2835-95-2
amino-4 hydroxy-2 toluene

| Conditions | Yield |
|---|---|
| With C43H37Br2CuN3P2(1+)*ClO4(1-) In methanol at 0 - 20℃; for 4h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 91% |
-
-
31643-49-9
4-Nitrophthalonitrile

-
-
2835-95-2
amino-4 hydroxy-2 toluene

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 65℃; for 6h; Inert atmosphere; | 90.8% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
305371-18-0
4-chloro-6-fluoro-[1,7]naphthyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| In ethanol for 16h; | 90% |
| In ethanol for 16h; | 90% |
-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
475585-11-6
4-chloro-6-(4-nitrophenyl)furo[2,3-d]pyrimidine

-
-
475585-19-4
2-methyl-5-{[6-(4-nitrophenyl)furo[2,3-d]pyrimidin-4-yl]amino}phenol

| Conditions | Yield |
|---|---|
| In butan-1-ol for 3h; Heating; | 89% |
-
-
22445-41-6
3,5-dimethylphenyl iodide

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
1198117-28-0
3-(3,5-dimethylphenoxy)-4-methylaniline

| Conditions | Yield |
|---|---|
| With 2-Picolinic acid; potassium phosphate; copper(l) iodide In dimethyl sulfoxide at 80℃; for 24h; Inert atmosphere; | 87% |
-
-
105297-10-7
4-(4-aminophenyl)thiomorpholine 1,1-dioxide

-
-
2835-95-2
amino-4 hydroxy-2 toluene

| Conditions | Yield |
|---|---|
| Stage #1: 4-(4-aminophenyl)thiomorpholine 1,1-dioxide With hydrogenchloride; isopentyl nitrite In methanol; water; acetonitrile at 0℃; for 1h; Inert atmosphere; Stage #2: amino-4 hydroxy-2 toluene With potassium carbonate In methanol; water; acetonitrile at 0℃; for 1h; | 87% |
-
-
7461-50-9
2-chloropyrimidin-4-amine

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
933045-96-6
5-(4-aminopyrimidin-2-ylamino)-2-methylphenol

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloropyrimidin-4-amine; amino-4 hydroxy-2 toluene With hydrogenchloride In ethanol; water at 90℃; for 18h; Stage #2: With potassium carbonate In ethanol; water; ethyl acetate Product distribution / selectivity; | 86% |
| With hydrogenchloride In ethanol; water at 80 - 90℃; Product distribution / selectivity; |
-
-
352-33-0
1-Chloro-4-fluorobenzene

-
-
2835-95-2
amino-4 hydroxy-2 toluene

-
-
1198117-37-1
3-[(4-fluorophenyl)amino]-6-methylphenol

| Conditions | Yield |
|---|---|
| With chloro[2-(dicyclohexylphosphino)-3,6-dimethoxy-2',4',6'-triisopropyl-1,1'-biphenyl][2-(2-aminoethyl)phenyl]palladium(ll); sodium t-butanolate In 1,4-dioxane at 90℃; for 1.16667h; Inert atmosphere; | 86% |
5-Amino-o-cresol Chemical Properties
Structure of 5-Amino-o-cresol (CAS NO.2835-95-2):
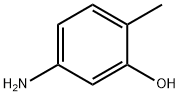
IUPAC Name: 5-amino-2-methylphenol
Empirical Formula: C7H9NO
Molecular Weight: 123.1525
EINECS: 220-618-6
Index of Refraction: 1.616
Molar Refractivity: 37.19 cm3
Molar Volume: 106.4 cm3
Polarizability: 14.74×10-24cm3
Surface Tension: 51.9 dyne/cm
Density: 1.157 g/cm3
Flash Point: 116.3 °C
Enthalpy of Vaporization: 52.71 kJ/mol
Melting Point: 160-162 °C(lit.)
Boiling Point: 268.6 °C at 760 mmHg
Vapour Pressure: 0.0046 mmHg at 25°C
Product Categories: Intermediates of Dyes and Pigments;Amines;blocks;Aromatic Hydrocarbons (substituted) & Derivatives;Aromatic Phenols;Phenol&Thiophenol&Mercaptan;Organic Chemicals
Synonyms of 5-Amino-o-cresol (CAS NO.2835-95-2): 2-Hydroxy-p-toluidine ; 3-Amino-6-methylphenol ; 3-Hydroxy-4-methylaniline ; 4-13-00-01666 (Beilstein Handbook Reference) ; 4-Amino-2-hydroxytoluene ; 5-Amino-2-methylphenol ; 6-Methyl-3-aminophenol
5-Amino-o-cresol Toxicity Data With Reference
| 1. | mmo-sat 333 µg/plate | EMMUEG Environmental and Molecular Mutagenesis. 11 (Suppl 12)(1988),1. | ||
| 2. | orl-rat LD50:3600 mg/kg | FCTXAV Food and Cosmetics Toxicology. 15 (1977),607. | ||
| 3. | orl-qal LD50:750 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 12 (1983),355. | ||
| 4. | orl-rat LD50:3600 mg/kg | FCTXAV Food and Cosmetics Toxicology. 15 (1977),607. |
5-Amino-o-cresol Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
5-Amino-o-cresol Safety Profile
Hazard Codes:  Xi,
Xi, Xn
Xn
Risk Statements: 36/37/38-20/21/22
R36/37/38:Irritating to eyes, respiratory system and skin.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 1
RTECS: SJ6090000
Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also PHENOL.
Related Products
- 5-Amino-o-cresol
- 2835-96-3
- 2835-97-4
- 2835-98-5
- 2835-99-6
- 2836-00-2
- 28361-10-6
- 2836-32-0
- 28363-79-3
- 2836-40-0
- 2836-44-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View