-
Name
5-Chlorovaleryl chloride
- EINECS 216-403-1
- CAS No. 1575-61-7
- Article Data29
- CAS DataBase
- Density 1.183 g/cm3
- Solubility reacts with water
- Melting Point
- Formula C5H8Cl2O
- Boiling Point 188.9 °C at 760 mmHg
- Molecular Weight 155.024
- Flash Point 76.3 °C
- Transport Information UN 3265 8/PG 2
- Appearance clear colourless to slightly yellow liquid
- Safety 26-36/37/39-45
- Risk Codes 22-34-23
-
Molecular Structure
-
Hazard Symbols
 C,
C, T
T
- Synonyms Valerylchloride, 5-chloro- (6CI,7CI,8CI);5-Chloropentanoyl chloride;5-Chlorovalericacid chloride;5-Chlorovaleroyl chloride;NSC 84182;
- PSA 17.07000
- LogP 2.16090
Synthetic route

| Conditions | Yield |
|---|---|
| With thionyl chloride for 3h; Heating / reflux; | 97% |
| With thionyl chloride for 3h; Heating / reflux; | 97% |
| With thionyl chloride for 3h; Heating / reflux; | 97% |
-
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
-
75-44-5
phosgene

-
-
108-99-6
3-Methylpyridine

-
-
1575-61-7
5-Chlorovaleroyl chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 81% |

-
-
638-29-9
n-valeryl chloride

-
A
-
61589-68-2
2-chloropentanoyl chloride

-
B
-
63480-12-6
4-chloropentanoyl chloride

-
C
-
1575-61-7
5-Chlorovaleroyl chloride

-
D
-
90631-23-5
3-chloro-valeryl chloride

| Conditions | Yield |
|---|---|
| With phenylchloroiodonium chloride In tetrachloromethane at 30℃; Product distribution; Mechanism; Irradiation; relative rate const., the correlation analysis, subst. phenylchloroiodonium chloride as reagents; | |
| With chlorine In neat (no solvent) Product distribution; Ambient temperature; liquid phase chlorination of the aliphatic C5-carboxylic acids and their chlorides, methyl esters and chloromethyl esters, relative reactivity of each hydrogen atoms; | |
| With sulfuryl dichloride; 2,2'-azobis(isobutyronitrile) In tetrachloromethane Heating; Title compound not separated from byproducts; |

-
-
638-29-9
n-valeryl chloride

-
A
-
63480-12-6
4-chloropentanoyl chloride

-
B
-
1575-61-7
5-Chlorovaleroyl chloride

-
C
-
90631-23-5
3-chloro-valeryl chloride

| Conditions | Yield |
|---|---|
| With chlorine In benzene at 20℃; Product distribution; other solvents; |

-
-
7782-50-5
chlorine

-
-
638-29-9
n-valeryl chloride

-
A
-
61589-68-2
2-chloropentanoyl chloride

-
B
-
63480-12-6
4-chloropentanoyl chloride

-
C
-
1575-61-7
5-Chlorovaleroyl chloride

-
D
-
90631-23-5
3-chloro-valeryl chloride

| Conditions | Yield |
|---|---|
| at 20℃; im UV-Licht; | |
| at 125℃; im UV-Licht; |

-
-
7782-50-5
chlorine

-
-
638-29-9
n-valeryl chloride

-
-
71-43-2
benzene

-
A
-
61589-68-2
2-chloropentanoyl chloride

-
B
-
63480-12-6
4-chloropentanoyl chloride

-
C
-
1575-61-7
5-Chlorovaleroyl chloride

-
D
-
90631-23-5
3-chloro-valeryl chloride

| Conditions | Yield |
|---|---|
| at 20℃; im UV-Licht; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: concentrated aqueous hydrochloric acid 2: thionyl chloride View Scheme |
-
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
-
75-44-5
phosgene

-
-
24044-24-4
1,3,4-trimethylimidazolidin-2-one

-
-
1575-61-7
5-Chlorovaleroyl chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride |


| Conditions | Yield |
|---|---|
| With hydrogenchloride |

-
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
-
75-44-5
phosgene

-
-
24307-26-4
mepiquat chloride

-
-
1575-61-7
5-Chlorovaleroyl chloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride |


| Conditions | Yield |
|---|---|
| With Dichloromethyl methyl ether; zinc(II) chloride at 60℃; for 2h; |
-
-
773837-37-9
sodium cyanide

-
-
110-56-5
1,4-dichlorobutane

-
A
-
1575-61-7
5-Chlorovaleroyl chloride

-
B
-
111-50-2
Adipic acid dichloride

| Conditions | Yield |
|---|---|
| Stage #1: sodium cyanide; 1,4-dichlorobutane With tetrabutylammomium bromide In water at 80 - 85℃; for 6h; Large scale; Stage #2: With hydrogenchloride In water at 55 - 70℃; for 5h; Large scale; Stage #3: With thionyl chloride at 20 - 30℃; for 18h; Large scale; | A 422 kg B 163 kg |

-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
144108-56-5
6,12-bis(benzyloxy)-7-oxo-6,12-diazadodecanenitrile

-
-
144108-57-6
17-chloro-6,12-bis(benzyloxy)-7,13-dioxo-6,12-diazaheptadecanenitrile

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane Ambient temperature; | 100% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
613662-01-4
5-fluoro-2-phenoxyaniline

-
-
613662-02-5
5-chloro-N-(5-fluoro-2-phenoxy-phenyl)-pentanamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20 - 25℃; for 1.5h; | 100% |
-
-
928630-93-7
5-chloro-thiophene-2-carboxylic acid-N-({1-[4-amino-3-methyl-phenyl]-1H-imidazol-4-yl}-methyl)-amide

-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
928630-94-8
5-chloro-thiophene-2-carboxylic acid-N-({1-[4-({4-chloro-butyl-carbonyl}-amino)-3-methyl-phenyl]-1H-imidazol-4-yl}-methyl)-amide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 16h; | 100% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
23218-93-1
3-amino-5-nitro-benzoic acid methyl ester

-
-
706791-79-9
3-(4-chloropentanoylamino)-5-nitrobenzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 1h; | 100% |
-
-
75-09-2
dichloromethane

-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
21835-63-2
1,4-dihydro-4-(3-aminophenyl)-2,6-dimethyl-3,5-pyridinedicarboxylic acid,dimethyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water; ethyl acetate | 100% |
| In tetrahydrofuran; water; ethyl acetate | 100% |
| In tetrahydrofuran; water; ethyl acetate | 100% |

-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
1037235-89-4
[cis-4-amino-1-(3-chlorophenyl)-cyclohexylmethyl]-carbamic acid tert butyl ester

-
-
1037197-18-4
C23H34Cl2N2O3

| Conditions | Yield |
|---|---|
| With sodium carbonate In chloroform; water at 20℃; for 0.75h; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 0 - 20℃; for 1h; | 100% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
1372187-09-1
(6R,7R)-tert-butyl 6-(aminomethyl)-7-(4-chloro-3-fluorophenyl)-1,4-oxazepane-4-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 100% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
2133-40-6, 65365-28-8, 79397-50-5
methyl pyrrolidine-2-carboxylate hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 1h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 18h; Inert atmosphere; | 99% |
| Stage #1: m-Anisidine With sodium hydride In tetrahydrofuran; mineral oil for 6h; Inert atmosphere; Stage #2: 5-Chlorovaleroyl chloride In tetrahydrofuran; mineral oil at 20℃; for 6h; Inert atmosphere; | 72% |
-
-
182502-68-7
SCP-1

-
-
1575-61-7
5-Chlorovaleroyl chloride

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In acetonitrile at 20℃; for 1h; Friedel-Crafts Acylation; | 99% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
98454-40-1, 98454-41-2
(1S,3S)-3-Benzyloxy-cyclohexylamine

-
-
98454-46-7, 98454-47-8
5-Chloro-pentanoic acid ((1S,3S)-3-benzyloxy-cyclohexyl)-amide

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran; water for 0.5h; Ambient temperature; | 98% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
100-66-3
methoxybenzene

-
-
949-06-4
5-chloro-1-(4-methyloxyphenyl)pentan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 5-Chlorovaleroyl chloride With aluminum (III) chloride In dichloromethane at 0℃; for 0.166667h; Stage #2: methoxybenzene In dichloromethane at 0 - 20℃; for 1h; | 98% |
| With aluminium trichloride Acylation; | 69% |
-
-
288-32-4
1H-imidazole

-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
929453-35-0
1-(5-chloro-pentanoyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 18 - 25℃; | 98% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 4 - 20℃; for 2h; | 98% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
54166-95-9
2-amino-6-chlorobenzamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 97% |
| With tetrabutylammomium bromide; sodium hydroxide In dichloromethane; water at 25 - 30℃; for 1h; Reagent/catalyst; | 97.84% |
| Inert atmosphere; | |
| Schotten-Baumann reaction; chemoselective reaction; | |
| With triethylamine In tetrahydrofuran |
-
-
1575-61-7
5-Chlorovaleroyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 2h; Cooling with ice; | 97.8% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin(IV) chloride In water; benzene | 97.7% |

| Conditions | Yield |
|---|---|
| Stage #1: 5-Chlorovaleroyl chloride; aniline With tetrabutylammomium bromide; sodium hydroxide In dichloromethane; water at 0 - 5℃; for 1h; Stage #2: With potassium hydroxide In dichloromethane; water at 20℃; Reagent/catalyst; Temperature; Solvent; | 97.36% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 10 - 25℃; Inert atmosphere; | 97.3% |
-
-
1575-61-7
5-Chlorovaleroyl chloride


| Conditions | Yield |
|---|---|
| With potassium carbonate In water; acetone at -5 - 0℃; for 0.5h; Large scale; | 97.1% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
76801-94-0
5-amino-N,N'-bis[2,3-bis(acetyloxy)propyl]2,4,6-triiodo-1,3-benzenedicarboxamide

-
-
136453-24-2
N,N'-Bis[2,3-bis(Acetyloxy)propyl]-5-[(5-chloro-1-oxo-pentyl)amino]-2,4,6-triiodo-1,3-benzenedicarboxamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide for 30h; Ambient temperature; | 97% |

-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
76801-94-0
5-amino-N,N'-bis[2,3-bis(acetyloxy)propyl]2,4,6-triiodo-1,3-benzenedicarboxamide

-
B
-
136453-24-2
N,N'-Bis[2,3-bis(Acetyloxy)propyl]-5-[(5-chloro-1-oxo-pentyl)amino]-2,4,6-triiodo-1,3-benzenedicarboxamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl acetamide; ethyl acetate | A 97% B n/a |

-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
380383-75-5
tert-butyl 1-(4-methoxyphenyl)hydrazine-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In dichloromethane; water at 0 - 20℃; | 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 97% |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
1267610-26-3
1-(4-aminophenyl)-3-(morpholin-4-yl)-5,6-dihydropyridin-2(1H)-one

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium hydroxide In dichloromethane; water at 0 - 5℃; for 1h; Reagent/catalyst; | 96.08% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 10 - 25℃; Inert atmosphere; | 89.5% |
| With triethylamine In tetrahydrofuran at 5 - 50℃; for 2h; Inert atmosphere; |
-
-
1575-61-7
5-Chlorovaleroyl chloride

-
-
96695-11-3
1-methoxy-2-phenyl-1-(trimethylsiloxy)but-1-ene

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; titanium tetrachloride In dichloromethane at 20 - 25℃; for 1.5h; Claisen condensation; | 96% |
| With pentafluorophenylammonium triflate In dichloromethane at 60℃; for 1.5h; | 229 mg |
5-Chlorovaleryl chloride Specification
The 5-Chlorovaleryl chloride, with the CAS registry number 1575-61-7 and EINECS registry number 216-403-1, is a kind of clear colourless to slightly yellow liquid. It belongs to the product categories of Miscellaneous Biochemicals and Acid Chlorides. And the molecular formula of the chemical is C5H8Cl2O.
The physical properties of 5-Chlorovaleryl chloride are as followings: (1)ACD/LogP: 1.87; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.87; (4)ACD/LogD (pH 7.4): 1.87; (5)ACD/BCF (pH 5.5): 15.56; (6)ACD/BCF (pH 7.4): 15.56; (7)ACD/KOC (pH 5.5): 248.26; (8)ACD/KOC (pH 7.4): 248.26; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.446; (14)Molar Refractivity: 34.94 cm3; (15)Molar Volume: 130.9 cm3; (16)Polarizability: 13.85×10-24cm3; (17)Surface Tension: 32.6 dyne/cm; (18)Density: 1.183 g/cm3; (19)Flash Point: 76.3 °C; (20)Enthalpy of Vaporization: 42.51 kJ/mol; (21)Boiling Point: 188.9 °C at 760 mmHg; (22)Vapour Pressure: 0.586 mmHg at 25°C.
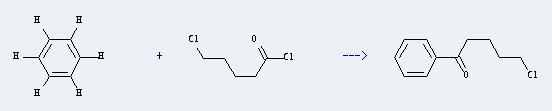
Uses of 5-Chlorovaleryl chloride: It can react with benzene to produce 5-chloro-1-phenyl-pentan-1-one. This reaction will need reagent AlCl3. The reaction time is 0.5 hours with temperature of 0°C, and the yield is about 89%.
You should be cautious while dealing with this chemical. It is harmful if swallowed, and toxic by inhalation. It may also cause burns. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice, or in case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: ClCCCCC(Cl)=O
(2)InChI: InChI=1/C5H8Cl2O/c6-4-2-1-3-5(7)8/h1-4H2
(3)InChIKey: SVNNWKWHLOJLOK-UHFFFAOYAK
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 320mg/kg (320mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#02242, |
Related Products
- 5-Chlorovaleryl chloride
- 15756-57-7
- 157577-99-6
- 157590-59-5
- 157596-04-8
- 157596-41-3
- 1575-96-8
- 15760-35-7
- 15760-36-8
- 157604-46-1
- 157606-25-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View