-
Name
1-[[4-[2-(azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methyl-indol-5-ol
- EINECS 805-732-1
- CAS No. 198481-32-2
- Article Data34
- CAS DataBase
- Density 1.19 g/cm3
- Solubility
- Melting Point 98-102°
- Formula C30H34N2O3
- Boiling Point 694.4 °C at 760 mmHg
- Molecular Weight 470.612
- Flash Point 373.8 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms TSE-424;Bazedoxifene [INN];Bazedoxifeno;1-((4-(2-Hexahydro-1H-azepin-1-yl)ethoxy)phenyl)methyl)-2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol;1H-Indol-5-ol, 1-((4-(2-(hexahydro-1H-azepin-1-yl)ethoxy)phenyl)methyl)-2-(4-hydroxyphenyl)-3-methyl-;Bazedoxifeno [INN-Spanish];1-[[4-[2-(Azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methyl-indol-5-ol;
- PSA 57.86000
- LogP 6.26890
Synthetic route
-
-
198480-21-6
5-benzyloxy-2-(4-benzyloxy-phenyl)-3-methyl-1-[4-(2-azepan-1-yl-ethoxy)-benzyl]-1H-indole

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; sodium hydroxide In ethanol; ethyl acetate Solvent; Reagent/catalyst; | 100% |
| With hydrogen; 2.34% Pd/C In tetrahydrofuran; ethanol for 5h; | 99% |
| With hydrogen; palladium 10% on activated carbon In methanol; water; acetone at 40℃; under 7355.72 Torr; Product distribution / selectivity; | 99.72% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With trimethylsilyl iodide In chloroform at 20℃; for 5h; | 95% |
| With hydrogen iodide | 36.3 g |
-
-
223251-25-0
1-{2-[4-(chloromethyl)phenoxy]ethyl}hexahydro-1H-azepine hydrochloride

-
-
91444-55-2
4-(5-acetoxy-3-methyl-1H-indol-2-yl)phenyl acetate

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Stage #1: 4-(5-acetoxy-3-methyl-1H-indol-2-yl)phenyl acetate With sodium hydride In N,N-dimethyl-formamide at 0 - 5℃; for 2h; Inert atmosphere; Stage #2: 1-{2-[4-(chloromethyl)phenoxy]ethyl}hexahydro-1H-azepine hydrochloride In N,N-dimethyl-formamide at 0 - 25℃; for 15h; Stage #3: With sodium hydroxide In N,N-dimethyl-formamide at 25℃; for 15h; Solvent; Temperature; Reagent/catalyst; | 89% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 20℃; for 0.5h; | 87% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; water for 5h; Reflux; | 86% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With ammonium formate; 10 wt% Pd(OH)2 on carbon In acetone | 85% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With ammonia In methanol; water at 20℃; for 3h; | 85% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate at 45℃; for 3h; | 83% |
-
-
1251936-45-4
1-(2-{4-[5-Hydroxy-2-(4-hydroxy-phenyl)-3-methyl-indol-1-ylmethyl]-phenoxy}-ethyl)-azepane-2,7-dione

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; boron trifluoride diethyl etherate In tetrahydrofuran at 5 - 10℃; for 4h; | 80.7% |
-
-
111-49-9
hexamethylene imine

-
-
1343413-19-3
2-(4-((5-hydroxy-2-(4-hydroxyphenyl)-3-methyl-1H-indol-1-yl)methyl)phenoxy)ethyl 4-methylbenzenesulfonate

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| In toluene at 70 - 75℃; for 12h; | 80% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 20℃; for 3h; | 79% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; water pH=9.5 - 10.5; Product distribution / selectivity; | 73.27% |
-
-
223251-25-0
1-{2-[4-(chloromethyl)phenoxy]ethyl}hexahydro-1H-azepine hydrochloride

-
-
91444-54-1
2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Stage #1: 1-{2-[4-(chloromethyl)phenoxy]ethyl}hexahydro-1H-azepine hydrochloride; 2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol With sodium hydride In N,N-dimethyl-formamide at 0℃; for 1h; Stage #2: 1-{2-[4-(chloromethyl)phenoxy]ethyl}hexahydro-1H-azepine hydrochloride In N,N-dimethyl-formamide at 0 - 5℃; for 3.91h; | 70.18% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With boron tribromide In methanol for 5h; Reflux; | 69% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid In methanol at 50℃; for 2h; | 61% |
-
-
1072-21-5
hexanedial

-
-
1251936-44-3
1-[4-(2-Amino-ethoxy)-benzyl]-2-(4-hydroxy-phenyl)-3-methyl-1H-indol-5-ol

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| 10 wt% Pd(OH)2 on carbon at 45 - 50℃; under 3677.86 - 5884.58 Torr; | 45% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With acetyl chloride In methanol at 0 - 2℃; for 3h; | 42% |

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane at 20℃; for 20h; | 15% |
-
-
623-05-2
(4-hydroxyphenyl)methanol

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: K2CO3 2.1: SOCl2 / tetrahydrofuran 3.1: sodium hydride / dimethylformamide / 0.33 h / 0 °C 3.2: 59 percent / dimethylformamide / 18 h / 20 °C 4.1: 78 percent / LiAlH4 / tetrahydrofuran / 0.5 h / 0 °C 5.1: 87 percent / CBr4; Ph3P / tetrahydrofuran / 3 h / 20 °C 6.1: tetrahydrofuran 7.1: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydroxide / tetrabutylammomium bromide / toluene; water / Reflux 2.1: hydrogenchloride / dichloromethane / 25 - 35 °C / Inert atmosphere 3.1: sodium hydride / N,N-dimethyl-formamide; toluene / -5 - -1 °C 3.2: 5 °C 4.1: hydrogen / palladium 10% on activated carbon / water; acetone; methanol / 40 °C / 7355.72 Torr View Scheme | |
| Multi-step reaction with 7 steps 1.1: potassium carbonate / acetonitrile / 60 °C 2.1: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / 0.75 h / 0 °C / Inert atmosphere 3.1: sodium hydride / N,N-dimethyl-formamide / 0.33 h / 0 °C 3.2: 20 °C 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 0.5 h / 0 °C 5.1: carbon tetrabromide; triphenylphosphine / tetrahydrofuran / 20 °C 6.1: tetrahydrofuran / Reflux 7.1: palladium 10% on activated carbon; hydrogen / tetrahydrofuran; ethanol / 20 °C View Scheme |
-
-
35081-45-9
1-<4-(benzyloxy)phenyl>-2-bromo-1-propanone

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 51 percent / Et3N / dimethylformamide / Heating 2.1: sodium hydride / dimethylformamide / 0.33 h / 0 °C 2.2: 59 percent / dimethylformamide / 18 h / 20 °C 3.1: 78 percent / LiAlH4 / tetrahydrofuran / 0.5 h / 0 °C 4.1: 87 percent / CBr4; Ph3P / tetrahydrofuran / 3 h / 20 °C 5.1: tetrahydrofuran 6.1: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / N,N-dimethyl-formamide / 100 - 125 °C 2.1: sodium hydride / N,N-dimethyl-formamide; toluene / -5 - -1 °C 2.2: 5 °C 3.1: hydrogen / palladium 10% on activated carbon / water; acetone; methanol / 40 °C / 7355.72 Torr View Scheme | |
| Multi-step reaction with 6 steps 1.1: triethylamine / N,N-dimethyl-formamide / 4 h / 120 - 150 °C / Inert atmosphere 2.1: sodium hydride / N,N-dimethyl-formamide / 0.33 h / 0 °C 2.2: 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 0.5 h / 0 °C 4.1: carbon tetrabromide; triphenylphosphine / tetrahydrofuran / 20 °C 5.1: tetrahydrofuran / Reflux 6.1: palladium 10% on activated carbon; hydrogen / tetrahydrofuran; ethanol / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / N,N-dimethyl-formamide / 4 h / 115 °C 2: sodium hydride / N,N-dimethyl-formamide / 10 h / 20 °C 3: boron trifluoride diethyl etherate / dichloromethane; ethanethiol / 4 h / 0 - 25 °C View Scheme |
-
-
51388-20-6
4-benzyloxyaniline hydrochloride

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 51 percent / Et3N / dimethylformamide / Heating 2.1: sodium hydride / dimethylformamide / 0.33 h / 0 °C 2.2: 59 percent / dimethylformamide / 18 h / 20 °C 3.1: 78 percent / LiAlH4 / tetrahydrofuran / 0.5 h / 0 °C 4.1: 87 percent / CBr4; Ph3P / tetrahydrofuran / 3 h / 20 °C 5.1: tetrahydrofuran 6.1: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / N,N-dimethyl-formamide / 100 - 125 °C 2.1: sodium hydride / N,N-dimethyl-formamide; toluene / -5 - -1 °C 2.2: 5 °C 3.1: hydrogen / palladium 10% on activated carbon / water; acetone; methanol / 40 °C / 7355.72 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: triethylamine / N,N-dimethyl-formamide / 4 h / 115 °C 2: sodium hydride / N,N-dimethyl-formamide / 10 h / 20 °C 3: boron trifluoride diethyl etherate / dichloromethane; ethanethiol / 4 h / 0 - 25 °C View Scheme |
-
-
80494-75-3
ethyl [p-(chloromethyl)phenoxy]acetate

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium hydride / dimethylformamide / 0.33 h / 0 °C 1.2: 59 percent / dimethylformamide / 18 h / 20 °C 2.1: 78 percent / LiAlH4 / tetrahydrofuran / 0.5 h / 0 °C 3.1: 87 percent / CBr4; Ph3P / tetrahydrofuran / 3 h / 20 °C 4.1: tetrahydrofuran 5.1: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 0.33 h / 0 °C 1.2: 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 0.5 h / 0 °C 3.1: carbon tetrabromide; triphenylphosphine / tetrahydrofuran / 20 °C 4.1: tetrahydrofuran / Reflux 5.1: palladium 10% on activated carbon; hydrogen / tetrahydrofuran; ethanol / 20 °C View Scheme |
-
-
103258-64-6
ethyl 2-(4-hydroxymethylphenoxy)acetate

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: SOCl2 / tetrahydrofuran 2.1: sodium hydride / dimethylformamide / 0.33 h / 0 °C 2.2: 59 percent / dimethylformamide / 18 h / 20 °C 3.1: 78 percent / LiAlH4 / tetrahydrofuran / 0.5 h / 0 °C 4.1: 87 percent / CBr4; Ph3P / tetrahydrofuran / 3 h / 20 °C 5.1: tetrahydrofuran 6.1: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / 0.75 h / 0 °C / Inert atmosphere 2.1: sodium hydride / N,N-dimethyl-formamide / 0.33 h / 0 °C 2.2: 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 0.5 h / 0 °C 4.1: carbon tetrabromide; triphenylphosphine / tetrahydrofuran / 20 °C 5.1: tetrahydrofuran / Reflux 6.1: palladium 10% on activated carbon; hydrogen / tetrahydrofuran; ethanol / 20 °C View Scheme |
-
-
198479-63-9
5-benzyloxy-2-(4-benzyloxy-phenyl)-3-methyl-1H-indole

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium hydride / dimethylformamide / 0.33 h / 0 °C 1.2: 59 percent / dimethylformamide / 18 h / 20 °C 2.1: 78 percent / LiAlH4 / tetrahydrofuran / 0.5 h / 0 °C 3.1: 87 percent / CBr4; Ph3P / tetrahydrofuran / 3 h / 20 °C 4.1: tetrahydrofuran 5.1: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium hydride / N,N-dimethyl-formamide; toluene / -5 - -1 °C 1.2: 5 °C 2.1: hydrogen / palladium 10% on activated carbon / water; acetone; methanol / 40 °C / 7355.72 Torr View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 0.33 h / 0 °C 1.2: 20 °C 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 0.5 h / 0 °C 3.1: carbon tetrabromide; triphenylphosphine / tetrahydrofuran / 20 °C 4.1: tetrahydrofuran / Reflux 5.1: palladium 10% on activated carbon; hydrogen / tetrahydrofuran; ethanol / 20 °C View Scheme |
-
-
198479-95-7
2-(4-((5-(benzyloxy)-2-(4-(benzyloxy)phenyl)-3-methyl-1H-indol-1-yl)methyl)phenoxy)ethanol

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 87 percent / CBr4; Ph3P / tetrahydrofuran / 3 h / 20 °C 2: tetrahydrofuran 3: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: carbon tetrabromide; triphenylphosphine / tetrahydrofuran / 20 °C 2: tetrahydrofuran / Reflux 3: palladium 10% on activated carbon; hydrogen / tetrahydrofuran; ethanol / 20 °C View Scheme |
-
-
198480-07-8
5-benzyloxy-2-(4-benzyloxy-phenyl)-1-[4-(2-bromo-ethoxy)-benzyl]-3-methyl-1H-indole

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrahydrofuran 2: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: tetrahydrofuran / Reflux 2: palladium 10% on activated carbon; hydrogen / tetrahydrofuran; ethanol / 20 °C View Scheme |
-
-
198479-82-2
{4-[5-benzyloxy-2-(4-benzyloxy-phenyl)-3-methyl-indol-1-ylmethyl]-phenoxy}-acetic acid ethyl ester

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 78 percent / LiAlH4 / tetrahydrofuran / 0.5 h / 0 °C 2: 87 percent / CBr4; Ph3P / tetrahydrofuran / 3 h / 20 °C 3: tetrahydrofuran 4: H2 / Pd/C / ethanol; tetrahydrofuran / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: lithium aluminium tetrahydride / tetrahydrofuran / 0.5 h / 0 °C 2: carbon tetrabromide; triphenylphosphine / tetrahydrofuran / 20 °C 3: tetrahydrofuran / Reflux 4: palladium 10% on activated carbon; hydrogen / tetrahydrofuran; ethanol / 20 °C View Scheme |
-
-
1049816-47-8
C30H36N2O9P2

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With water; alkaline phosphatase pH=7.4; Conversion of starting material; Tris buffer; Enzymatic reaction; |
-
-
223251-16-9
[4-(2-hexamethyleneimino-1-yl-ethoxy)-phenyl]-methanol

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogenchloride / dichloromethane / 25 - 35 °C / Inert atmosphere 2.1: sodium hydride / N,N-dimethyl-formamide; toluene / -5 - -1 °C 2.2: 5 °C 3.1: hydrogen / palladium 10% on activated carbon / water; acetone; methanol / 40 °C / 7355.72 Torr View Scheme |

| Conditions | Yield |
|---|---|
| for 6h; Heating; | 98.5% |
| In ethanol; ethyl acetate at 20 - 30℃; | 95.5% |
| In acetone at 20 - 55℃; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 4h; Inert atmosphere; | 93.48% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 4h; Inert atmosphere; | 90.3% |
-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol Product distribution / selectivity; | 87.79% |
| With hydrogenchloride In methanol; water |

| Conditions | Yield |
|---|---|
| Stage #1: L-Lactic acid In ethanol at 60℃; for 0.25h; Stage #2: bazedoxifene In ethanol at 60℃; for 0.333333h; Solvent; | 66.4% |
-
-
802294-64-0
propionic acid

-
-
198481-32-2
bazedoxifene

-
-
1266685-12-4
1-[4-(2-azepan-1-yl-ethoxy)benzyl]-2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol propionate

| Conditions | Yield |
|---|---|
| In acetone | 60.23% |
-
-
64-18-6
formic acid

-
-
198481-32-2
bazedoxifene

| Conditions | Yield |
|---|---|
| In ethyl acetate at 77℃; | 50% |
Bazedoxifene Specification
The Bazedoxifene, with the CAS registry number 198481-32-2, is also known as 1-{4-[2-(Azepan-1-yl)ethoxy]benzyl}-2-(4-hydroxyphenyl)-3-methyl-1H-indol-5-ol. This chemical's molecular formula is C30H34N2O3 and molecular weight is 470.60. What's more, its IUPAC name is 1-[[4-[2-(Azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methylindol-5-ol. In addition, this chemical's classification code are Antiestrogen; Estrogen Receptor Modulator. Besides, it can be used as a kind of potential drugs in the prevention and treatment of postmenopausal osteoporosis. And Bazedoxifene's combination with conjugated estrogens, Aprela, is currently undergoing Phase III studies.
Physical properties about Bazedoxifene are: (1)ACD/LogP: 6.44; (2)# of Rule of 5 Violations: 1; (3)#H bond acceptors: 5; (4)#H bond donors: 2; (5)#Freely Rotating Bonds: 9; (6)Polar Surface Area: 35.86 Å2; (7)Index of Refraction: 1.622; (8)Molar Refractivity: 138.88 cm3; (9)Molar Volume: 394.1 cm3; (10)Polarizability: 55.05×10-24 cm3; (11)Surface Tension: 46.5 dyne/cm; (12)Density: 1.19 g/cm3; (13)Flash Point: 373.8 °C; (14)Enthalpy of Vaporization: 105.37 kJ/mol; (15)Boiling Point: 694.4 °C at 760 mmHg; (16)Vapour Pressure: 6.33E-20 mmHg at 25 °C.
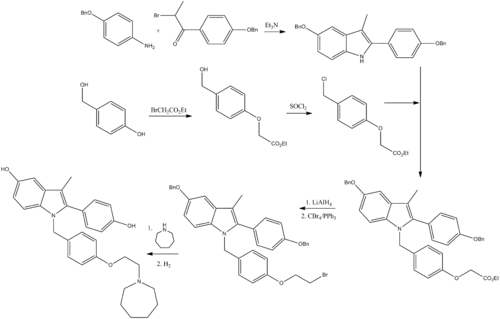
Preparation of Bazedoxifene: this chemical is prepared by the following synthetic steps
You can still convert the following datas into molecular structure:
(1) SMILES: Oc1ccc(cc1)c3c(c2cc(O)ccc2n3Cc5ccc(OCCN4CCCCCC4)cc5)C
(2) InChI: InChI=1/C30H34N2O3/c1-22-28-20-26(34)12-15-29(28)32(30(22)24-8-10-25(33)11-9-24)21-23-6-13-27(14-7-23)35-19-18-31-16-4-2-3-5-17-31/h6-15,20,33-34H,2-5,16-19,21H2,1H3
(3) InChIKey: UCJGJABZCDBEDK-UHFFFAOYAW
Related Products
- Bazedoxifene
- 19848-67-0
- 198490-77-6
- 198491-04-2
- 1985-12-2
- 19852-25-6
- 19853-09-9
- 19853-10-2
- 19853-64-6
- 19853-79-3
- 198541-90-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View