-
Name
4-CYANOTHIOPHENOL
- EINECS
- CAS No. 36801-01-1
- Article Data16
- CAS DataBase
- Density 1.18 g/cm3
- Solubility
- Melting Point 51-52℃
- Formula C7H5NS
- Boiling Point 264.4 °C at 760 mmHg
- Molecular Weight 135.189
- Flash Point 113.7 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-Cyanobenzenethiol;4-Cyanothiophenol;4-Mercaptobenzonitrile;p-Cyanobenzenethiol;p-Cyanothiophenol;4-sulfanylbenzonitrile;
- PSA 62.59000
- LogP 1.84698
Synthetic route
-
-
623-00-7
4-bromobenzenecarbonitrile

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromobenzenecarbonitrile With caesium carbonate; copper(II) sulfate In dimethyl sulfoxide for 0.166667h; Inert atmosphere; Stage #2: With ethane-1,2-dithiol In dimethyl sulfoxide at 100℃; for 20h; Inert atmosphere; | 100% |
| With copper(ll) sulfate pentahydrate; caesium carbonate; ethane-1,2-dithiol In dimethyl sulfoxide at 110℃; for 20h; Inert atmosphere; | 87% |
| Multi-step reaction with 2 steps 1: 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; tris-(dibenzylideneacetone)dipalladium(0); N-ethyl-N,N-diisopropylamine / toluene / 14 h / 120 °C / Inert atmosphere 2: sodium methylate / methanol; mineral oil / 14 h / 0 - 20 °C View Scheme | |
| Stage #1: 4-bromobenzenecarbonitrile With TurboGrignard In tetrahydrofuran at 0℃; for 2h; Inert atmosphere; Stage #2: With sulfur In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; |
-
-
3058-39-7
4-iodobenzonitrile

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate; caesium carbonate; ethane-1,2-dithiol In dimethyl sulfoxide at 90℃; for 20h; Inert atmosphere; | 93% |
| With copper(l) iodide; thiourea; L-proline; sodium t-butanolate In dimethyl sulfoxide at 90℃; for 24h; Inert atmosphere; | 90% |
-
-
59177-46-7
4-mercaptobenzamide

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With trichlorophosphate In N,N-dimethyl-formamide at 0℃; for 2h; | 93% |

-
A
-
519-73-3
triphenylmethane

-
B
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With 2-methyl-N-cyclohexylpropanamide In N,N-dimethyl-formamide Mechanism; Rate constant; Thermodynamic data; electrolyse (Hg pool electrode); ΔG(excit.)0; other reagents (azobenzenes) with other electrode; | A 84% B 90% C 6% |

-
-
19290-43-8
4-cyanophenyl-S,N,N-dimethyl thiocarbamate

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In tetrahydrofuran; methanol at 20℃; for 3h; | 84% |
| With potassium hydroxide In tetrahydrofuran; methanol for 3h; Ambient temperature; | 81% |
| With potassium hydroxide In methanol |
-
-
150993-53-6
4-(benzylsulfanyl)benzonitrile

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In benzene at 20℃; for 144h; Inert atmosphere; | 76% |
-
-
1194-02-1
4-fluorobenzonitrile

-
A
-
36801-01-1
4-cyanobenzenethiol

-
B
-
6339-51-1
bis(4-cyanophenyl)disulfane

-
C
-
46836-99-1
bis(4-cyanophenyl)sulfide

| Conditions | Yield |
|---|---|
| With potassium sulfide In N,N-dimethyl-formamide at 50 - 55℃; for 21h; | A 5% B 73% C 9.5% |
| With potassium sulfide In N,N-dimethyl-formamide at 50 - 55℃; for 21h; | A 5% B 73% C 9.5% |
| With potassium sulfide In N,N-dimethyl-formamide at 50 - 55℃; for 21h; | A 5% B 73% C 9.5% |

-
-
1194-02-1
4-fluorobenzonitrile

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| Stage #1: 4-fluorobenzonitrile With sodium sulfide In N,N-dimethyl-formamide at 20℃; for 36h; Inert atmosphere; Stage #2: With hydrogenchloride; zinc In water Cooling with ice; Inert atmosphere; | 46% |
| Stage #1: 4-fluorobenzonitrile With sodium sulfide In N,N-dimethyl-formamide at 20℃; Stage #2: With hydrogenchloride; zinc In water at 0℃; for 1h; | 18.2% |
-
-
623-03-0
4-Cyanochlorobenzene

-
-
17356-08-0
thiourea

-
A
-
36801-01-1
4-cyanobenzenethiol

-
B
-
6339-51-1
bis(4-cyanophenyl)disulfane

-
C
-
46836-99-1
bis(4-cyanophenyl)sulfide

| Conditions | Yield |
|---|---|
| With 4,4'-bipyridine; potassium tert-butylate In ammonia at -40℃; Rate constant; electrochemical reaction; | A 34% B 13% C 14% |
| With 4,4'-bipyridine; potassium tert-butylate In ammonia at -40℃; electrolysis; | A 34% B 13% C 14% |


-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water | 30% |
-
-
67-56-1
methanol

-
-
1194-02-1
4-fluorobenzonitrile

-
A
-
6302-65-4
methyl 4-mercaptobenzoate

-
B
-
36801-01-1
4-cyanobenzenethiol

-
C
-
46836-99-1
bis(4-cyanophenyl)sulfide

| Conditions | Yield |
|---|---|
| With potassium sulfide; sulfuric acid; zinc Yield given. Multistep reaction; | A n/a B 5% C 9.5% |


-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With buffer (pH=11) at 20℃; | |
| With buffer (pH=11) at 20℃; Rate constant; |

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| Stage #1: trifluoro-acetic acid 4-cyano-phenylsulfanylmethyl ester With ethanol; triethylamine Stage #2: With ammonium chloride |
-
-
60958-04-5, 121405-10-5, 97474-48-1
4-methanesulfinylbenzonitrile

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| Stage #1: 4-methanesulfinylbenzonitrile With trifluoroacetic anhydride for 0.5h; Heating; Stage #2: With triethylamine In methanol | 219 mg |
| Multi-step reaction with 2 steps 1.1: Heating 2.1: Et3N; EtOH 2.2: NH4Cl View Scheme |
-
-
21382-98-9
p-cyanophenyl methyl sulfide

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: mCPBA / 0 °C 2.1: Heating 3.1: Et3N; EtOH 3.2: NH4Cl View Scheme | |
| Multi-step reaction with 2 steps 1.1: meta-chloroperoxybenzoic acid / CHCl3 / 2 h / 0 °C 2.1: trifluoroacetic anhydride / 0.5 h / Heating 2.2: 219 mg / triethylamine / methanol View Scheme |
-
-
141212-58-0
5-p-(p-bromophenylsulphonyl)phenyl-3-mercapto-1,2,4-triazole

-
A
-
79995-40-7
4-((4-bromophenyl)thio)benzonitrile

-
B
-
80988-12-1
4-((4-bromophenyl)sulfonyl)benzonitrile

-
C
-
139-66-2
diphenyl sulfide

-
D
-
23038-36-0
1-bromo-4-(phenylsulphonyl)benzene

-
E
-
3393-78-0
4,4'-dibromodiphenyl sulfide

-
F
-
36801-01-1
4-cyanobenzenethiol

-
G
-
106-53-6
para-bromobenzenethiol

| Conditions | Yield |
|---|---|
| at 475℃; under 2 Torr; Pyrolysis; Inert atmosphere; | A 3.6 %Chromat. B 50 %Chromat. C 1.3 %Chromat. D 11.3 %Chromat. E 15.6 %Chromat. F 2.2 %Chromat. G 5.9 %Chromat. |

-
-
141212-52-4
(phenylsulphonyl)phenyl-3-mercapto-1,2,4-triazole

-
A
-
139-66-2
diphenyl sulfide

-
B
-
127-63-9
diphenyl sulphone

-
C
-
28525-13-5
4-(phenylsulfonyl)benzonitrile

-
D
-
55204-60-9
4-benzenesulfonyl-benzoic acid amide

-
E
-
36801-01-1
4-cyanobenzenethiol

-
F
-
108-98-5
thiophenol

| Conditions | Yield |
|---|---|
| at 475℃; under 2 Torr; Pyrolysis; Inert atmosphere; | A 0.4 %Chromat. B 6.4 %Chromat. C 67.1 %Chromat. D 5.7 %Chromat. E 0.6 %Chromat. F 5.9 %Chromat. |

-
-
20994-09-6
4-cyanophenyl-O,N,N-dimethyl thiocarbamate

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 200 °C / Inert atmosphere 2: potassium hydroxide / methanol; tetrahydrofuran / 3 h / 20 °C View Scheme |
-
-
767-00-0
4-cyanophenol

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1,4-diaza-bicyclo[2.2.2]octane / N,N-dimethyl-formamide / 65 °C 2: 200 °C / Inert atmosphere 3: potassium hydroxide / methanol; tetrahydrofuran / 3 h / 20 °C View Scheme |

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol; mineral oil at 0 - 20℃; for 14h; | 1.79 g |
-
-
623-03-0
4-Cyanochlorobenzene

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydride / mineral oil; N,N-dimethyl-formamide / 0.5 h / 10 °C / Inert atmosphere 1.2: 14 h / 20 °C / Inert atmosphere 2.1: aluminum (III) chloride / benzene / 144 h / 20 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 4-Aminobenzonitrile With hydrogenchloride In water at 0℃; for 0.166667h; Stage #2: With sodium nitrite In water at 0℃; for 1h; Stage #3: potassium ethyl xanthogenate Further stages; |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
2746-25-0
p-Methoxybenzyl bromide

-
-
1257086-86-4
4-[(4-methoxybenzyl)thio]benzonitrile

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20 - 80℃; for 18h; | 100% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
49584-26-1
p-cyanobenzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; isopropyl alcohol In dichloromethane at 0 - 20℃; for 1h; | 99% |
-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In 1,2-dichloro-ethane at 0℃; for 0.5h; | 97% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
316376-13-3
1,3-di-O-acetyl-2,5-anhydro-4-deoxy-6-thio-α-D-xylo-hexoseptanose

-
-
316376-15-5
4-cyanophenyl 3-O-acetyl-2,5-anhydro-4-deoxy-1,6-dithio-α-D-xylo-hexoseptanoside

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In 1,2-dichloro-ethane at 20℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 16h; | 96.85% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
6339-51-1
bis(4-cyanophenyl)disulfane

| Conditions | Yield |
|---|---|
| With TiO2/MoS2 (10:1 molar ratio of Ti to Mo) nanocomposite; air In ethanol at 20℃; Irradiation; Green chemistry; | 95% |
| With hydrogen bromide; dimethyl sulfoxide In chloroform at 20℃; for 8h; Inert atmosphere; Schlenk technique; | 91% |
| With dimethyl sulfoxide at 65℃; | 78% |
| With iodine; potassium iodide | |
| With iodine |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
354150-48-4
methyl 1,3,4,5-tetra-O-acetyl-2,6-anhydro-D-mannose hemiacetal

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In 1,2-dichloro-ethane at -10℃; for 0.5h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-cyanobenzenethiol With potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.333333h; Williamson ether synthesis; Stage #2: 1,5-dibromo-pentane In N,N-dimethyl-formamide at 130 - 140℃; Williamson ether synthesis; | 94% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
100-00-5
4-chlorobenzonitrile

-
-
21969-10-8
4-cyanophenyl 4′-nitrophenyl sulfide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; Inert atmosphere; | 93% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
189382-65-8
1,5-anhydro-3,4-di-O-benzoyl-5-thio-D-threo-pent-1-enitol

-
-
189382-68-1
4-cyanophenyl 3,4-di-O-benzoyl-2-deoxy-1,5-dithio-D-threo-pentopyranoside

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-anhydro-3,4-di-O-benzoyl-5-thio-D-threo-pent-1-enitol With hydrogen bromide In toluene at 10℃; for 0.333333h; Addition; Stage #2: With silver(I) acetate In toluene; acetonitrile at 20℃; for 2h; Substitution; Stage #3: 4-cyanobenzenethiol With trimethylsilyl trifluoromethanesulfonate In dichloromethane; toluene; acetonitrile at -10 - 20℃; for 1h; Condensation; | 91% |
-
-
22731-83-5
S,S-diphenylsulphoximine

-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With Cu(4-N,N-dimethylaminopyridine)4I; oxygen In dichloromethane at 20℃; | 91% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
4837-25-6
4-((difluoromethyl)thio)benzonitrile

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; tris[2-phenylpyridinato-C2,N]iridium(III) In acetonitrile at 20℃; for 48h; Schlenk technique; Sealed tube; Inert atmosphere; Irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile | 90% |

| Conditions | Yield |
|---|---|
| With nanolayered cobalt-molybdenum sulphide with Co/(Mo+Co) mole ratio 0.83 In Hexadecane; toluene at 180℃; under 2625.26 Torr; for 18h; Inert atmosphere; Autoclave; chemoselective reaction; | 90% |
-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 4h; Inert atmosphere; Reflux; | 89% |
| With hydrogenchloride; oxalic acid In water at 20℃; for 0.75h; Kinetics; UV-irradiation; chemoselective reaction; | 93 %Chromat. |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
109-70-6
1,3-chlorobromopropane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 89% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
316376-25-7
1,4-di-O-acetyl-2,5-anhydro-3-O-methyl-6-thio-α-L-guloseptanose

-
-
316376-27-9
4-cyanophenyl 4-O-acetyl-2,5-anhydro-3-O-methyl-1,6-dithio-α-L-guloseptanoside

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In 1,2-dichloro-ethane at 20℃; for 1h; | 88% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
59647-83-5
1-O-acetyl-2,5-anhydro-3,4-di-O-methanesulfonyl-6-thio-α-D-glucoseptanose

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In 1,2-dichloro-ethane at 20℃; for 1h; | 88% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran AgOAc and PPh3 dissolved in THF; HSC6H4CN added; soln. stirred for 2 h; layered with hexane for 3 d; elem. anal.; | 88% |


-
-
36801-01-1
4-cyanobenzenethiol

-
-
1299398-65-4
4-(quinolin-3-ylsulfanyl)benzonitrile

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; ethylene glycol In N,N-dimethyl acetamide at 110℃; for 48h; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium 3-(diphenylphosphanyl)benzenesulfonate In water at 120℃; for 16h; Sealed tube; Green chemistry; | 86% |
| With dichloro bis(acetonitrile) palladium(II) In water at 120℃; for 16h; Sealed tube; | 69% |
| With sulfuric acid In acetic acid |
-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With copper(I) thiophene-2-carboxylate; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 19h; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide | 85% |
-
-
36801-01-1
4-cyanobenzenethiol

-
-
321157-02-2
1,5-di-O-acetyl-2,6-anhydro-3-O-(4-nitrobenzoyl)-2-thio-α-D-altrofuranose

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In 1,2-dichloro-ethane at 20℃; for 1h; | 85% |
-
-
36801-01-1
4-cyanobenzenethiol

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In 1,2-dichloro-ethane at 20℃; for 24h; | 85% |
Benzonitrile,4-mercapto- Specification
The Benzonitrile,4-mercapto-, with the CAS registry number 36801-01-1, has the systematic name of 4-sulfanylbenzonitrile. It belongs to the product categories of Thiol and Aromatic Nitriles. And the molecular formula of the chemical is C7H5NS.
The characteristics of Benzonitrile,4-mercapto- are as followings: (1)ACD/LogP: 2.32; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.74; (4)ACD/LogD (pH 7.4): 0.03; (5)ACD/BCF (pH 5.5): 8.89; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 113.68; (8)ACD/KOC (pH 7.4): 2.23; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 49.09 Å2; (13)Index of Refraction: 1.602; (14)Molar Refractivity: 39.16 cm3; (15)Molar Volume: 114 cm3; (16)Polarizability: 15.52×10-24cm3; (17)Surface Tension: 50.6 dyne/cm; (18)Density: 1.18 g/cm3; (19)Flash Point: 113.7 °C; (20)Enthalpy of Vaporization: 50.23 kJ/mol; (21)Boiling Point: 264.4 °C at 760 mmHg; (22)Vapour Pressure: 0.00972 mmHg at 25°C.
Uses of Benzonitrile,4-mercapto-: It can react with thiocarbonyl dichloride to produce (p-Cyanphenyl)chlordithioformiat. This reaction will need reagent NaOH, and the yield is about 85%.
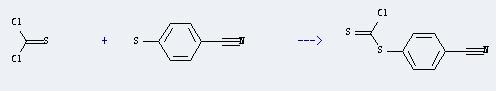
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: N#Cc1ccc(S)cc1
(2)InChI: InChI=1/C7H5NS/c8-5-6-1-3-7(9)4-2-6/h1-4,9H
(3)InChIKey: MVPUXVBBHWUOFS-UHFFFAOYAD
Related Products
- Benzonitrile
- Benzonitrile, 2,4-dichloro-6-methyl-
- Benzonitrile, 2,4-dimethoxy-6-methyl-
- Benzonitrile, 2,6-dinitro-
- Benzonitrile, 2-[(6-chloro-3,4-dihydro-2,4-dioxo-1(2H)-pyrimidinyl)methyl]-
- Benzonitrile, 2-amino-4-nitro-
- Benzonitrile, 2-chloro-3-formyl-
- Benzonitrile, 2-chloro-4-fluoro-3-methyl-
- Benzonitrile, 2-chloro-6-methoxy-
- Benzonitrile, 2-ethyl-
- 36802-41-2
- 36804-17-8
- 36805-97-7
- 3680-69-1
- 3680-71-5
- 36809-26-4
- 36809-75-3
- 36810-87-4
- 36810-90-9
- 368-16-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View