-
Name
Bisphenol A
- EINECS 201-245-8
- CAS No. 80-05-7
- Article Data243
- CAS DataBase
- Density 1.195 g/cm3
- Solubility 120–300 ppm (at 21.5 °C) in water
- Melting Point 158 to 159 °C (430 K)
- Formula C15H16O2
- Boiling Point 220 °C (493 K) / 4 mmHg
- Molecular Weight 228.291
- Flash Point 227 °C
- Transport Information
- Appearance White to light brown flakes or powder
- Safety 26-36/37/39-45-46
- Risk Codes 37-41-43-62
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 4,4'-(propane-2,2-diyl)diphenol;p,p'-isopropylidenebisphenol;BPA;4,4'-(1-Methylethylidene)bisphenol;2,2-(4,4'-Dihydroxydiphenyl)propane;2,2-Bis(4-hydroxyphenyl)propane;4,4'-Bisphenol A;4,4'-Isopropylidenediphenol;2,2-Di(4-phenylol)propane;
- PSA 40.46000
- LogP 3.42370
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; triethylamine In methanol; water at 120℃; under 22502.3 Torr; for 116h; Autoclave; | 95% |
| With hydrogen; triethylamine In ethanol; water at 120℃; under 22502.3 Torr; for 116h; Autoclave; | 95% |
| With cadmium selenide; triethylamine In N,N-dimethyl-formamide at 20℃; for 24h; Irradiation; Sealed tube; | 87% |
| With sodium sulfite In water at 130℃; for 12h; Sealed tube; Microwave irradiation; Green chemistry; | 80% |

| Conditions | Yield |
|---|---|
| With silica supported perchloric acid In neat (no solvent) for 3.75h; Heating; Green chemistry; | 88% |
| With magnetic mesoporous silica supported azacrown ether hexafluorophosphate ionic liquid In water at 50℃; for 5h; Temperature; Green chemistry; | 86% |
| sulfonic-acid-form cation-exchange resin (DIAION "SK104H") modified with hydrolyzed 2-pyridylethyl thioacetate at 70℃; for 2h; Product distribution / selectivity; Inert atmosphere; Cooling; | 58% |

| Conditions | Yield |
|---|---|
| In chlorobenzene; acetone | 83% |


| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 81% |


| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 81% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride at 40℃; for 48h; | 80% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 78% |


-
-
80-05-7
BPA

| Conditions | Yield |
|---|---|
| With iron(III) chloride; potassium phosphate; tetrabutylammomium bromide; N,N`-dimethylethylenediamine In water at 180℃; under 5250.53 Torr; for 20h; | 76% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,2'-[isopropylidenebis(1,4-phenyleneoxy)]diacetic acid With triethylamine In N,N-dimethyl-formamide; toluene for 3h; Curtius rearrangement; Heating; Stage #2: With potassium hydroxide; glycerol In ethanol; N,N-dimethyl-formamide; toluene for 2h; Heating; Further stages.; | 72% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 68% |

-
-
67-64-1
acetone

-
-
108-95-2
phenol

-
A
-
80-05-7
BPA

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
837-08-1
2-[1-(4-hydroxyphenyl)-1-methylethyl]-phenol

| Conditions | Yield |
|---|---|
| beta zeolite acidic form at 120℃; under 760.051 Torr; for 12h; | A 63.2% B 0.01% C 11.43% |


| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 80℃; for 12h; Solvent; Temperature; Green chemistry; | A 62% B 58% |


| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 62% |


| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 60% |


| Conditions | Yield |
|---|---|
| With uranyl nitrate hydrate; water; trifluoroacetic acid In nitromethane for 48h; Irradiation; Inert atmosphere; | 60% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 52% |

-
-
67-64-1
acetone

-
-
108-95-2
phenol

-
A
-
80-05-7
BPA

-
B
-
7559-72-0
2,2-bis(2-hydroxyphenyl)-propane

-
C
-
5026-12-0
2-(2-hydroxyphenyl)-2,4,4-trimethylchroman

-
D
-
63661-69-8
4'-hydroxy-2,4,4-trimethylflavan

-
E
-
837-08-1
2-[1-(4-hydroxyphenyl)-1-methylethyl]-phenol

| Conditions | Yield |
|---|---|
| With aluminium(III) phenoxide at 140 - 160℃; for 1h; Product distribution; oth. temperature, oth. ratio of reactants, solvents; | A 8.1% B 1.5% C 7% D 21.6% E 50.3% |


| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 50% |


| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A 48% B n/a |


| Conditions | Yield |
|---|---|
| In ethanol; water at 80℃; for 12h; Green chemistry; | A n/a B 28% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanethiol | |
| With sulfuric acid; ethanethiol |

| Conditions | Yield |
|---|---|
| With boron trifluoride |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With sulfuric acid | |
| With hydrogenchloride | |
| With hydrogenchloride; zinc(II) chloride |
-
-
80-05-7
BPA

-
-
13804-54-1, 13804-57-4, 13804-58-5, 80-04-6
4,4'-isopropylidenedicyclohexanol

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water | 100% |
| With 10% Ru/C; hydrogen In isopropyl alcohol at 90℃; under 3800.26 Torr; for 21h; | 74% |
| With kieselguhr; ethanol; nickel at 180 - 200℃; under 51485.6 - 128714 Torr; Hydrogenation.Isolierung durch fraktionierte Krystallisation aus Aethanol-Benzol-Gemischen und aus Aceton; |

| Conditions | Yield |
|---|---|
| at 200℃; for 2h; | 100% |
-
-
80-05-7
BPA

-
-
56-81-5
glycerol

-
-
5581-32-8
3-[4-[1-[4-(2,3-dihydroxypropoxy)phenyl]-1-methylethyl]phenoxy]propane-1,2-diol

| Conditions | Yield |
|---|---|
| With potassium carbonate; Diethyl carbonate at 110℃; for 18h; | 100% |
-
-
80-05-7
BPA

-
-
38184-64-4
4,4'-(propane-2,2-diyl)bis(4,1-phenylene) disulfofluoridate

| Conditions | Yield |
|---|---|
| Stage #1: BPA With triethylamine In dichloromethane at 20℃; for 0.166667h; Stage #2: With fluorosulfonyl fluoride In dichloromethane at 20℃; Sealed tube; | 100% |
| Stage #1: BPA With triethylamine In dichloromethane at 20℃; for 0.166667h; Stage #2: With fluorosulfonyl fluoride In dichloromethane at 20℃; Sealed tube; | 100% |
| With fluorosulfonyl fluoride; triethylamine In dichloromethane at 20℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| at 150℃; for 8h; Inert atmosphere; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 145℃; for 3h; | 65% |

| Conditions | Yield |
|---|---|
| With potassium tribromide In water; acetonitrile at 30℃; for 0.0833333h; | 99% |
| With hydrogenchloride; sodium bromate; sodium dodecyl-sulfate; sodium bromide In tetrachloromethane; water at 10℃; for 4.5h; | 98.28% |
| With dihydrogen peroxide; bromine In dichloromethane; water | 96% |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 20℃; for 96h; | 99% |
| With methanesulfonic acid at 25℃; for 96h; | 95% |
| With methanesulfonic acid at 20℃; for 96h; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: BPA With potassium carbonate In acetone for 1h; Inert atmosphere; Reflux; Stage #2: allyl bromide In acetone Reflux; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| In diethylene glycol dimethyl ether at 80℃; for 5h; | 99% |

| Conditions | Yield |
|---|---|
| In diethylene glycol dimethyl ether at 80℃; for 5h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: BPA With potassium carbonate In sulfolane; toluene at 20℃; for 0.75h; Stage #2: 2-chloroquinoxaline at 80℃; for 48h; Further stages.; | 99% |
-
-
80-05-7
BPA

-
-
589-15-1
1-bromomethyl-4-bromobenzene

-
-
159639-83-5
4,4'-(isopropylidenediphenyl)-bis(4-bromobenzyl) ether

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 99% |
-
-
80-05-7
BPA

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
139725-20-5
2,2-bis(4-trifluoromethanesulfonyloxyphenyl)propane

| Conditions | Yield |
|---|---|
| Stage #1: BPA With pyridine In dichloromethane at 0℃; for 0.166667h; Inert atmosphere; Stage #2: trifluoromethylsulfonic anhydride In dichloromethane at 0℃; Inert atmosphere; | 98.5% |
| With pyridine for 25h; Ambient temperature; | 96% |
| With pyridine at 0 - 20℃; | 93% |
-
-
80-05-7
BPA

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: BPA With 1H-imidazole In dichloromethane at 20℃; for 0.166667h; Stage #2: tert-butyldimethylsilyl chloride In dichloromethane at 20℃; for 24.5h; | 98.5% |
| With 1H-imidazole In dichloromethane at 20℃; Sealed tube; | 98% |
| With 1H-imidazole; dmap In dichloromethane; N,N-dimethyl-formamide for 3h; | 96% |
| With 1H-imidazole In dichloromethane at 20℃; | 93% |
| With 1H-imidazole In dichloromethane |
-
-
80-05-7
BPA

-
-
199334-40-2
3,3-bis[4-(4-fluorobenzoyl)phenyl]phthalide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl acetamide at 185℃; for 10h; Polymerization; | 98.5% |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl acetamide; potassium carbonate In chlorobenzene at 185℃; for 50h; | 98.5% |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl acetamide; potassium carbonate In chlorobenzene at 185℃; for 10h; | 98.5% |
-
-
80-05-7
BPA

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
103915-73-7
2,2-bis[4-(2-methylallyloxy)phenyl]propane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20 - 30℃; for 6h; | 98.5% |
| Stage #1: BPA With potassium hydroxide In ethanol Stage #2: 3-Chloro-2-methylpropene In ethanol for 10h; Reflux; | 10.32 g |

| Conditions | Yield |
|---|---|
| Stage #1: BPA; 3-Chloro-2-methylpropene With sodium hydroxide In water at 50 - 60℃; for 12h; pH=8 - 10; Stage #2: With hydrogen bromide; dihydrogen peroxide In water at 27 - 29℃; for 6h; Concentration; | 98.3% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; triethylamine In water; acetone | 98.1% |
| With hydrogenchloride; triethylamine In water; acetone | 98.1% |
| With triethylamine; isopropyl alcohol In acetone at -15 - -10℃; for 2h; Temperature; | 94% |
| With triethylamine In acetone at -30 - 20℃; for 1.5h; | 80% |
| With triethylamine In acetone at -5 - 0℃; |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium hydroxide at 20℃; for 12h; | 98% |
| Stage #1: BPA With sodium hydride In N,N-dimethyl-formamide at 20℃; for 0.25h; Inert atmosphere; Stage #2: epichlorohydrin In N,N-dimethyl-formamide at 20℃; for 18h; | 72% |
| Stage #1: BPA; epichlorohydrin With N-benzyl-N,N,N-triethylammonium chloride at 80℃; for 12h; Stage #2: With sodium hydroxide In water for 5h; | 47% |
-
-
80-05-7
BPA

-
-
1636-14-2
bis(diethylamino)phenylphosphine

-
-
146733-97-3
4,4′-(propane-2,2-diyl)di(benzene-4,1-diyl) bis(N,N-diethyl-P-phenylphosphonamidite)

| Conditions | Yield |
|---|---|
| at 115 - 120℃; a) 2 h, b) 10 mm Hg, 2 h, c) 1 mm Hg, 2 h; | 98% |
| at 115 - 120℃; for 1.5h; |

| Conditions | Yield |
|---|---|
| at 115 - 120℃; a) 2 h, b) 10 mm Hg, 2 h, c) 1 mm Hg, 2 h; | 98% |
| In xylene for 14h; Heating; | 85% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium iodide In ethanol for 24h; Heating; | 98% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydroxide at 40℃; for 6h; | 70% |
| With sodium hydroxide; calcium oxide In tert-butyl alcohol at 80℃; for 6h; Solvent; Reagent/catalyst; Temperature; | |
| Stage #1: BPA With sodium hydroxide In ethanol at 78℃; for 8h; Stage #2: 3-chloroprop-1-ene at 70℃; for 6h; |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0) In tetrahydrofuran at 25℃; for 24h; Condensation; | 98% |
| With potassium carbonate In neat (no solvent) at 85℃; for 4h; Catalytic behavior; | 98% |
-
-
80-05-7
BPA

-
-
106-96-7
propargyl bromide

-
-
22235-02-5
4,4'-(propane-2,2-diyl)bis((prop-2-ynyloxy)benzene)

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 22h; Heating; | 98% |
| Stage #1: BPA With sodium hydride In ethanol; N,N-dimethyl-formamide at -10 - 0℃; for 1.5h; Williamson Ether Synthesis; Inert atmosphere; Stage #2: propargyl bromide With tetra-(n-butyl)ammonium iodide In ethanol; N,N-dimethyl-formamide at 0 - 20℃; for 2.5h; Williamson Ether Synthesis; Inert atmosphere; | 92% |
| Stage #1: BPA With sodium hydroxide In water at 70℃; Stage #2: propargyl bromide With tetrabutylammomium bromide In water; toluene at 70 - 90℃; | 90% |

| Conditions | Yield |
|---|---|
| In diethylene glycol dimethyl ether at 70 - 80℃; for 4h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide; toluene at 140 - 145℃; for 6h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1-methyl-pyrrolidin-2-one; toluene at 150 - 155℃; for 6h; | 98% |
-
-
685561-32-4
2,2-bis[4-(3-hydroxyphenyl)phenyl]propane

-
-
80-05-7
BPA

-
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane at 0 - 15℃; for 1h; | 98% |

Bisphenol A Specification
Bisphenol A, with the CAS register number 80-05-7, is commonly abbreviated as BPA. Its EINECS register number is 201-245-8. Bisphenol A has IUPAC name which is called 4,4'-(propane-2,2-diyl)diphenol. The substance is an organic compound with the formula C15H16O2. It is a white to light brown flakes or powder with a weak medicine odor. Bisphenol A should be sealed and stored in cool and dry place. What's more, it should be protected from water and explosions.Bisphenol A, incompatible with strong oxidizers, strong bases, acid chlorides and acid anhydrides, will sink in water. Bisphenol A is combustible which may form explosive dust clouds. Bisphenol A is an endocrine disruptor, which can mimic the body's own hormones and may lead to negative health effects. September 2010, Canada became the first country to declare BPA as a toxic substance. In the European Union and Canada, BPA use is banned in baby bottles.
Physical properties about Bisphenol A are: (1)ACD/LogP: 3.641; (2)ACD/LogD (pH 5.5): 3.64; (3)ACD/LogD (pH 7.4): 3.64; (4)ACD/BCF (pH 5.5): 344.43; (5)ACD/BCF (pH 7.4): 343.99; (6)ACD/KOC (pH 5.5): 2278.55; (7)ACD/KOC (pH 7.4): 2275.67; (8)#H bond acceptors: 2; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 4; (11)Index of Refraction: 1.599; (12)Molar Refractivity: 68.168 cm3; (13)Molar Volume: 199.565 cm3; (14)Polarizability: 27.024 10-24cm3; (15)Surface Tension: 46.023998260498 dyne/cm; (16)Density: 1.144 g/cm3; (17)Flash Point: 192.423 °C; (18)Enthalpy of Vaporization: 67.716 kJ/mol; (19)Boiling Point: 400.837 °C at 760 mmHg;
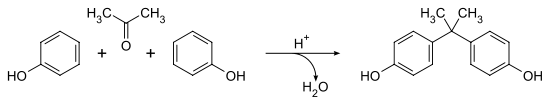
Production of Bisphenol A: Bisphenol A can be prepared by the condensation of acetone with two equivalents of phenol. The reaction is catalyzed by a strong acid, such as hydrochloric acid (HCl) or a sulfonated polystyrene resin. It is commonly that a large excess of phenol is used to ensure full condensation in industry. The product mixture of the cumene process may also be used as starting material.
Uses of Bisphenol A: Bisphenol A is used to make polycarbonate plastic and epoxy resins. Bisphenol A is primarily used to make plastics. It is a key monomer in production of epoxy resins and in the most common form of polycarbonate plastic which is clear and nearly shatter-proof and is used to make a variety of common products including baby and water bottles, sports equipment, medical and dental devices, dental fillings and sealants, eyeglass lenses, CDs and DVDs, and household electronics. As an antioxidant in some plasticizers, and as a polymerization inhibitor in PVC, Bisphenol A is also used in the synthesis of polysulfones and polyether ketones. Bisphenol A is also a precursor to the flame retardant tetrabromobisphenol A, and was formerly used as a fungicide. BPA-based products are also used in foundry castings and for lining water pipes.
When you are using Bisphenol A, you should be very cautious about it for its harmful. It is irritating to respiratory system. There will be a risk of serious damage to eyes and possible risk of impaired fertility. In addition, it may cause sensitisation by skin contact. In case of contact with eyes, you must rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately. If swallowed, you can seek medical advice immediately and show this container or label.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC(C)(C1=CC=C(C=C1)O)C2=CC=C(C=C2)O
(2)InChI: InChI=1S/C15H16O2/c1-15(2,11-3-7-13(16)8-4-11)12-5-9-14(17)10-6-12/h3-10,16-17H,1-2H3
(3)InChIKey: IISBACLAFKSPIT-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 4gm/kg (4000mg/kg) | AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 33(7), Pg. 25, 1968. |
| mammal (species unspecified) | LD50 | oral | 6500mg/kg (6500mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 135, 1969. | |
| mouse | LC | inhalation | > 1700mg/m3/2H (1700mg/m3) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 2, Pg. 50, 1961. |
| mouse | LD50 | intraperitoneal | 150mg/kg (150mg/kg) | National Technical Information Service. Vol. AD691-490, | |
| mouse | LD50 | oral | 2400mg/kg (2400mg/kg) | AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 33(7), Pg. 25, 1968. |
| mouse | LDLo | subcutaneous | 2500mg/kg (2500mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LIVER: FATTY LIVER DEGERATION | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 2, Pg. 50, 1961. |
| rabbit | LD50 | oral | 2230mg/kg (2230mg/kg) | American Industrial Hygiene Association Journal. Vol. 28, Pg. 301, 1967. | |
| rabbit | LD50 | skin | 3mL/kg (3mL/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 4, Pg. 119, 1951. | |
| rat | LD50 | oral | 3250mg/kg (3250mg/kg) | American Industrial Hygiene Association Journal. Vol. 28, Pg. 301, 1967. |
Related Products
- Bisphenol A
- Bisphenol A bis(2-hydroxyethyl)ether
- Bisphenol A bisallyl ether
- Bisphenol A diglycidyl ether
- BISPHENOL A DIGLYCIDYL ETHER, BROMINATED
- Bisphenol A diphosphate
- Bisphenol A disodium salt
- Bisphenol A epichlorohydrin polymer
- BISPHENOL A ETHOXYLATE DIMETHACRYLATE
- Bisphenol A-glycidyl methacrylate
- 8005-76-3
- 8005-77-4
- 80058-84-0
- 80058-85-1
- 80058-93-1
- 80060-09-9
- 8006-28-8
- 8006-32-4
- 8006-34-6
- 8006-38-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View