-
Name
Cephaloridine
- EINECS
- CAS No. 50-59-9
- Article Data5
- CAS DataBase
- Density 1.3230 (rough estimate)
- Solubility >20g/L(21 oC)
- Melting Point 184°C
- Formula C19H17 N3 O4 S2
- Boiling Point
- Molecular Weight 415.494
- Flash Point
- Transport Information
- Appearance
- Safety Experimental poison by intracerebral route. Moderately toxic to humans by intravenous route. Moderately toxic experimentally by ingestion, subcutaneous, intraperitoneal, and intravenous routes. Human systemic effects by intravenous route: nausea or vomiting and fatty liver degeneration. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NOx and SOx.
- Risk Codes 42/43
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms Pyridinium,1-[[(6R,7R)-2-carboxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-,inner salt (9CI); Pyridinium,1-[[2-carboxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-,hydroxide, inner salt, (6R-trans)-; Pyridinium,1-[[2-carboxy-8-oxo-7-[2-(2-thienyl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-,hydroxide, inner salt (8CI); 7-[a-(2-Thienyl)acetamido]-3-(1-pyridylmethyl)-3-cephem-4-carboxylic acidbetaine; Aliporina; Ampligram; Cefaloridin; Cefaloridine; Ceflorin;Cepaloridin; Cepalorin; Ceph 87/4; Cephaloridin; Cephaloridine; Cephlodine;Ceporan; Ceporin; Ceporine; Cer; Cilifor; Deflorin; Faredina; Floridin;Glaxoridin; Intrasporin; Keflodin; Keflordin; Kefspor; Lilly 40602; Lloncefal;Loridine; Pyridinium,1-[[2-carboxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-,inner salt, (6R-trans)-; Sch 11527; Sefacin
- PSA 146.96000
- LogP 0.01100
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium chloride In water at 30℃; Equilibrium constant; |

-
-
85904-88-7
2,2,2-trichloroethyl (6R,7R)-N-<7-(thien-2-ylacetamido)ceph-3-em-3-ylmethyl>pyridinium chloride 4-carboxylate

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| With Deacidite FF ion-exchange resin; zinc(II) chloride; zinc 1.) formic acid, 22 deg C, 2 h; Multistep reaction; |

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| With water-d2 at 37℃; Kinetics; Isomerization; pD = 10.5; |

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| With water-d2 at 37℃; Kinetics; Isomerization; pD = 10.5; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 67.3 percent / 1.5 h / 20 °C 2: 72.6 percent / phosphorus trichloride / CH2Cl2; dimethylformamide / 1.5 h / 21 °C 3: 1.) Zn, ZnCl2; 2.) Deacidite FF ion-exchange resin / 1.) formic acid, 22 deg C, 2 h View Scheme |
-
-
39098-97-0
2-thienylacetic acid chloride

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) phosphorus oxychloride; 2.) dimethylacetamide / 1.) MeOH, 45-55 deg C; 2.) CH2Cl2, 0-5 deg C, 45 min 2: 67.3 percent / 1.5 h / 20 °C 3: 72.6 percent / phosphorus trichloride / CH2Cl2; dimethylformamide / 1.5 h / 21 °C 4: 1.) Zn, ZnCl2; 2.) Deacidite FF ion-exchange resin / 1.) formic acid, 22 deg C, 2 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 46.3 percent / N,N'-dicyclohexylcarbodiimide / CH2Cl2 / 1 h / 20 °C 2: 67.3 percent / 1.5 h / 20 °C 3: 72.6 percent / phosphorus trichloride / CH2Cl2; dimethylformamide / 1.5 h / 21 °C 4: 1.) Zn, ZnCl2; 2.) Deacidite FF ion-exchange resin / 1.) formic acid, 22 deg C, 2 h View Scheme |
-
-
85904-87-6
2,2,2-trichloroethyl (1S,6R,7R)-3-chloromethyl-7-(thien-2-ylacetamido)ceph-3-em-4-carboxylate 1-oxide

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 67.3 percent / 1.5 h / 20 °C 2: 72.6 percent / phosphorus trichloride / CH2Cl2; dimethylformamide / 1.5 h / 21 °C 3: 1.) Zn, ZnCl2; 2.) Deacidite FF ion-exchange resin / 1.) formic acid, 22 deg C, 2 h View Scheme |
-
-
33492-94-3
2,2,2-trichloroethyl (1S,6R,7R)-N-<7-(thien-2-ylacetamido)ceph-3-em-3-ylmethyl>pyridinium chloride 4-carboxylate 1-oxide

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 72.6 percent / phosphorus trichloride / CH2Cl2; dimethylformamide / 1.5 h / 21 °C 2: 1.) Zn, ZnCl2; 2.) Deacidite FF ion-exchange resin / 1.) formic acid, 22 deg C, 2 h View Scheme |
-
-
85904-84-3
2,2,2-trichloroethyl (1S,6R,7R)-7-amino-3-chloromethylceph-3-em-4-carboxylate 1-oxide

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 46.3 percent / N,N'-dicyclohexylcarbodiimide / CH2Cl2 / 1 h / 20 °C 2: 67.3 percent / 1.5 h / 20 °C 3: 72.6 percent / phosphorus trichloride / CH2Cl2; dimethylformamide / 1.5 h / 21 °C 4: 1.) Zn, ZnCl2; 2.) Deacidite FF ion-exchange resin / 1.) formic acid, 22 deg C, 2 h View Scheme |
-
-
33465-56-4
2,2,2-trichloroethyl (1S,6R,7R)-3-bromomethyl-7-formamidoceph-3-em-4-carboxylate 1-oxide

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1.) phosphorus oxychloride; 2.) dimethylacetamide / 1.) MeOH, 45-55 deg C; 2.) CH2Cl2, 0-5 deg C, 45 min 2: 67.3 percent / 1.5 h / 20 °C 3: 72.6 percent / phosphorus trichloride / CH2Cl2; dimethylformamide / 1.5 h / 21 °C 4: 1.) Zn, ZnCl2; 2.) Deacidite FF ion-exchange resin / 1.) formic acid, 22 deg C, 2 h View Scheme | |
| Multi-step reaction with 5 steps 1: 46.2 percent / conc. hydrochloric acid / tetrahydrofuran / 30 h / 6 °C 2: 46.3 percent / N,N'-dicyclohexylcarbodiimide / CH2Cl2 / 1 h / 20 °C 3: 67.3 percent / 1.5 h / 20 °C 4: 72.6 percent / phosphorus trichloride / CH2Cl2; dimethylformamide / 1.5 h / 21 °C 5: 1.) Zn, ZnCl2; 2.) Deacidite FF ion-exchange resin / 1.) formic acid, 22 deg C, 2 h View Scheme |
-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| With nitric acid In water | 46.7% |

| Conditions | Yield |
|---|---|
| at 280℃; |


| Conditions | Yield |
|---|---|
| at 215 - 334℃; |


| Conditions | Yield |
|---|---|
| at 160 - 170℃; |

| Conditions | Yield |
|---|---|
| at 150 - 270℃; |

| Conditions | Yield |
|---|---|
| at 330℃; |


| Conditions | Yield |
|---|---|
| at 230 - 235℃; |

| Conditions | Yield |
|---|---|
| at 250 - 400℃; |

-
-
50-59-9
cefaloridine

-
-
141-78-6
ethyl acetate

-
A
-
64-17-5
ethanol

-
B
-
75-07-0
acetaldehyde

-
C
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| at 300 - 400℃; |

-
-
50-59-9
cefaloridine

-
-
122-66-7
diphenyl hydrazine

-
-
71-43-2
benzene

-
A
-
62-53-3
aniline

-
B
-
1227476-15-4
Azobenzene


| Conditions | Yield |
|---|---|
| With potassium chloride In water at 30℃; Rate constant; Equilibrium constant; effects of sodium hydroxide; |


| Conditions | Yield |
|---|---|
| With potassium chloride In water at 30℃; |

| Conditions | Yield |
|---|---|
| In water at 30℃; Rate constant; Mechanism; pH 5.5-12.5, I = 1.0 M (KCl); |


| Conditions | Yield |
|---|---|
| In water at 30℃; Rate constant; Mechanism; pH 10.5-13.5, I = 1.0 M (KCl); |


| Conditions | Yield |
|---|---|
| In water at 30℃; Rate constant; Mechanism; pH 8.35-12.5, I = 1.0 M (KCl); |

-
-
50-59-9
cefaloridine

-
A
-
110-86-1
pyridine

-
B
-
151338-10-2
(2R)-2-<(R)-carboxylato<(thien-2-yl)acetylamino>methyl>-5,6-dihydro-5-methylidene-1,3-thiazine-4-carboxylate

| Conditions | Yield |
|---|---|
| at 37℃; for 1h; Product distribution; Rate constant; Mechanism; carbonate-buffer solution, pH 10.5; | |
| With water-d2 at 37℃; Kinetics; Isomerization; hydrolysis; pD = 10.5; |

-
-
50-59-9
cefaloridine

| Conditions | Yield |
|---|---|
| copper(II) ion In water at 30℃; Rate constant; Mechanism; further metal-ions, pH; | |
| With hydrogenchloride In water at 30℃; Rate constant; hydrolysis; | |
| With potassium chloride; 2-hydroxyethanethiol In water at 30℃; pH=10.10; Kinetics; | |
| With potassium chloride; hydroxide In water at 30℃; Kinetics; |

| Conditions | Yield |
|---|---|
| at 150 - 160℃; |

| Conditions | Yield |
|---|---|
| Nachweis von 2,4,4,6,6,8,8-Heptamethyl-non-1-en in dem erhaltenen Produkt; |
-
-
50-59-9
cefaloridine

-
-
115-11-7
isobutene

-
A
-
107-39-1
2,4,4-trimethyl-1-pentene

-
B
-
2437-52-7
3-tert-Butyl-2,4,4-trimethyl-pent-2-ene

| Conditions | Yield |
|---|---|
| at 300℃; weitere Produkte: Hexaisobutylen, Heptaisobutylen; |


| Conditions | Yield |
|---|---|
| at 150 - 160℃; |


| Conditions | Yield |
|---|---|
| at 150 - 160℃; |
Cephaloridine Chemical Properties
Molecular Formula: C19H17N3O4S2
Molar mass: 415.486 g/mol
EINECS: 200-052-6
Structure of Cephaloridine (50-59-9):
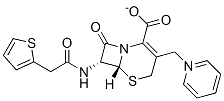
XLogP3-AA: 1.9
H-Bond Donor: 1
H-Bond Acceptor: 4
Systematic Name: (6R,7R)-8-oxo-3-(Pyridin-1-ium-1-ylmethyl)-7-[[2-(2-thienyl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
SMILES: O=C2N1/C(=C(\CS[C@@H]1[C@@H]2NC(=O)Cc3sccc3)C[n+]4ccccc4)C([O-])=O
InChI: InChI=1/C19H17N3O4S2/c23-14(9-13-5-4-8-27-13)20-15-17(24)22-16(19(25)26)12(11-28-18(15)22)10-21-6-2-1-3-7-21/h1-8,15,18H,9-11H2,(H-,20,23,25,26)/t15-,18-/m1/s1
InChIKey: CZTQZXZIADLWOZ-CRAIPNDOBC
Std. InChI: InChI=1S/C19H17N3O4S2/c23-14(9-13-5-4-8-27-13)20-15-17(24)22-16(19(25)26)12(11-28-18(15)22)10-21-6-2-1-3-7-21/h1-8,15,18H,9-11H2,(H-,20,23,25,26)/t15-,18-/m1/s1
Std. InChIKey: CZTQZXZIADLWOZ-CRAIPNDOSA-N
Cephaloridine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LD50 | intramuscular | > 200mg/kg (200mg/kg) | Toxicology and Applied Pharmacology. Vol. 8, Pg. 398, 1966. | |
| dog | LD | intravenous | > 1gm/kg (1000mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 863, 1965. |
| dog | LD50 | subcutaneous | > 200mg/kg (200mg/kg) | Toxicology and Applied Pharmacology. Vol. 8, Pg. 398, 1966. | |
| guinea pig | LD50 | subcutaneous | 550mg/kg (550mg/kg) | Toxicology and Applied Pharmacology. Vol. 8, Pg. 398, 1966. | |
| man | LDLo | intravenous | 1137mg/kg/4D (1137mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING LIVER: FATTY LIVER DEGERATION | JAMA, Journal of the American Medical Association. Vol. 200, Pg. 724, 1967. |
| monkey | LD50 | intramuscular | > 200mg/kg (200mg/kg) | Toxicology and Applied Pharmacology. Vol. 8, Pg. 398, 1966. | |
| mouse | LD50 | intracrebral | 10mg/kg (10mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" MUSCULOSKELETAL: CHANGES IN TEETH AND SUPPORTING STRUCTURES BEHAVIORAL: EXCITEMENT | Chemotherapy Vol. 26, Pg. 196, 1980. |
| mouse | LD50 | intramuscular | 7gm/kg (7000mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | Byoin Yakugaku. Hospital Pharmacology. Vol. 3, Pg. 220, 1978. |
| mouse | LD50 | intraperitoneal | 5355mg/kg (5355mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Japanese Journal of Antibiotics. Vol. 29, Pg. 735, 1976. |
| mouse | LD50 | intravenous | 2200mg/kg (2200mg/kg) | Toxicology and Applied Pharmacology. Vol. 8, Pg. 398, 1966. | |
| mouse | LD50 | oral | > 20gm/kg (20000mg/kg) | Japanese Journal of Antibiotics. Vol. 29, Pg. 735, 1976. | |
| mouse | LD50 | subcutaneous | 7745mg/kg (7745mg/kg) | Drugs in Japan Vol. -, Pg. 557, 1990. | |
| rabbit | LD50 | intramuscular | > 200mg/kg (200mg/kg) | Toxicology and Applied Pharmacology. Vol. 8, Pg. 398, 1966. | |
| rat | LD50 | intraperitoneal | 3170mg/kg (3170mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. | |
| rat | LD50 | intravenous | 1065mg/kg (1065mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: STRAUB TAIL | Japanese Journal of Antibiotics. Vol. 29, Pg. 735, 1976. |
| rat | LD50 | oral | 2500mg/kg (2500mg/kg) | Toxicology and Applied Pharmacology. Vol. 8, Pg. 398, 1966. | |
| rat | LD50 | parenteral | 2500ug/kg (2.5mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 863, 1965. | |
| rat | LD50 | subcutaneous | 2500mg/kg (2500mg/kg) | Toxicology and Applied Pharmacology. Vol. 8, Pg. 398, 1966. |
Cephaloridine Safety Profile
Experimental poison by intracerebral route. Moderately toxic to humans by intravenous route. Moderately toxic experimentally by ingestion, subcutaneous, intraperitoneal, and intravenous routes. Human systemic effects by intravenous route: nausea or vomiting and fatty liver degeneration. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NOx and SOx.
Cephaloridine Specification
Cephaloridine (50-59-9) also can be called Cefaloridine ; N-[7-(2'-Thienylacetamidoceph-3-ylmethyl)]pyridinium-2-carboxylate ; (6R,7R)-8-Oxo-3-(1-pyridiniumylmethyl)-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate ; (6R-trans)-1-[[2-Carboxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]pyridinium Hydroxide Inner Salt ; Pyridinium, 1-[[(6R,7R)-2-carboxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, inner salt ; and ; Cephaloridin .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View