-
Name
Cilostazol
- EINECS 689-122-9
- CAS No. 73963-72-1
- Article Data18
- CAS DataBase
- Density 1.34 g/cm3
- Solubility DMSO: 18 mg/mL, soluble
- Melting Point 159-160 °C
- Formula C20H27N5O2
- Boiling Point 664.7 °C at 760 mmHg
- Molecular Weight 369.467
- Flash Point 355.8 °C
- Transport Information
- Appearance off-white solid
- Safety
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 6-[4-(1-cyclohexyltetrazol-5-yl)butoxy]-3,4-dihydro-1H-quinolin-2-one;Pletal;OPC 13013;Cilostazol (JAN/USAN);2(1H)-Quinolinone,6-[4-(1-cyclohexyl-1Htetrazol- 5-yl)butoxy]-3,4-dihydro-;Pletal (TN);Cilostazole;Cilostal;
- PSA 81.93000
- LogP 3.60270
Synthetic route
-
-
877303-65-6
cilostazol bisulfate

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform; water Product distribution / selectivity; | 98.7% |
-
-
877303-63-4
cilostazol oxalate

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform; water Product distribution / selectivity; | 97.9% |
-
-
877303-64-5
cilostazol malate

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| With sodium hydroxide In chloroform; water Product distribution / selectivity; | 97.8% |

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); N-ethyl-N,N-diisopropylamine In acetonitrile for 10h; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; Reflux; | 96% |
-
-
54197-66-9
3,4-dihydro-6-hydroxy-2(1H)-quinolinone

-
-
73963-42-5
1-cyclohexyl-5-(4-chlorobutyl)-1,2,3,4-tetrazole

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| With potassium carbonate; sodium hydroxide; sodium sulfite In ethanol for 8h; Reflux; | 92.5% |
| With potassium carbonate; sodium hydroxide; sodium sulfite In water at 92℃; for 6h; Solvent; Reagent/catalyst; Temperature; | 91.5% |
| With potassium hydroxide In ethanol at 80℃; for 12h; Solvent; Reagent/catalyst; Temperature; Inert atmosphere; Sealed tube; | 90% |
-
-
54197-66-9
3,4-dihydro-6-hydroxy-2(1H)-quinolinone

-
-
73963-42-5
1-cyclohexyl-5-(4-chlorobutyl)-1,2,3,4-tetrazole

-
A
-
865792-18-3
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butoxy]-1-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butyl]-3,4-dihydro-1H-quinolin-2-one

-
B
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
C
-
73963-62-9
OPC 13015

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In ethanol at 75 - 80℃; | A n/a B 89% C n/a |


-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 25℃; for 8h; Inert atmosphere; | 88% |

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 25℃; for 8h; Inert atmosphere; | 85% |
-
-
73963-42-5
1-cyclohexyl-5-(4-chlorobutyl)-1,2,3,4-tetrazole

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| Stage #1: 3,4-dihydro-6-hydroxy-2(1H)-quinolinone With 1,8-diazabicyclo[5.4.0]undec-7-ene In ethanol for 6.5h; Heating / reflux; Molecular sieve; Stage #2: 1-cyclohexyl-5-(4-chlorobutyl)-1,2,3,4-tetrazole In ethanol for 6.5h; Product distribution / selectivity; Heating / reflux; | 40.2% |
| Multi-step reaction with 4 steps 1: sodium hydroxide / butan-1-ol / 8 h / Reflux 2: iron; ammonium chloride; acetic acid / methanol; water / 5 h / 45 °C / Reflux 3: triethylamine / dichloromethane / 3 h / 0 °C 4: N-ethyl-N,N-diisopropylamine; tetrakis(triphenylphosphine) palladium(0) / acetonitrile / 10 h / Inert atmosphere; Reflux View Scheme |
-
-
104-94-9
4-methoxy-aniline

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / acetone / 0.5 h 2: aluminum (III) chloride / N,N-dimethyl acetamide / 2 h / 150 °C 3: potassium hydroxide / ethanol / 12 h / 80 °C / Inert atmosphere; Sealed tube View Scheme |
-
-
5470-65-5
3-bromo-4-nitrophenol

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hydroxide / butan-1-ol / 8 h / Reflux 2: iron; ammonium chloride; acetic acid / methanol; water / 5 h / 45 °C / Reflux 3: triethylamine / dichloromethane / 3 h / 0 °C 4: N-ethyl-N,N-diisopropylamine; tetrakis(triphenylphosphine) palladium(0) / acetonitrile / 10 h / Inert atmosphere; Reflux View Scheme |

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: iron; ammonium chloride; acetic acid / methanol; water / 5 h / 45 °C / Reflux 2: triethylamine / dichloromethane / 3 h / 0 °C 3: N-ethyl-N,N-diisopropylamine; tetrakis(triphenylphosphine) palladium(0) / acetonitrile / 10 h / Inert atmosphere; Reflux View Scheme |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
144-62-7
oxalic acid

-
-
877303-63-4
cilostazol oxalate

| Conditions | Yield |
|---|---|
| In acetone at 20 - 25℃; for 3h; | 97% |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
877303-65-6
cilostazol bisulfate

| Conditions | Yield |
|---|---|
| With sulfuric acid In Isopropyl acetate at 20 - 25℃; for 3h; Product distribution / selectivity; | 94% |
| With sulfuric acid In acetone at 20 - 25℃; for 3h; | 92% |
| With sulfuric acid In butanone at 20 - 25℃; for 3h; Product distribution / selectivity; | 91% |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
110-16-7
maleic acid

-
-
877303-64-5
cilostazol malate

| Conditions | Yield |
|---|---|
| In acetone at 20 - 25℃; for 3h; | 90% |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
75-03-6
ethyl iodide

| Conditions | Yield |
|---|---|
| Stage #1: 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril With sodium hydride In mineral oil at 0℃; for 0.5h; Inert atmosphere; Stage #2: ethyl iodide In mineral oil at 0 - 20℃; for 12h; Inert atmosphere; | 81% |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
A
-
93632-84-9
OPC-13326

-
-
87153-03-5, 87153-05-7
3,4-Dihydro-6-<4-<1-(cis-2-hydroxycyclohexyl)-1H-tetrazol-5-yl>butoxy>-2(1H)-quinolinone

-
-
98360-32-8, 98360-33-9
3,4-Dihydro-6-<4-<1-(trans-3-hydroxycyclohexyl)-1H-tetrazol-5-yl>butoxy>-2(1H)-quinolinone

-
-
98360-32-8, 98360-33-9
3,4-Dihydro-6-<4-<1-(cis-3-hydroxycyclohexyl)-1H-tetrazol-5-yl>butoxy>-2(1H)-quinolinone

-
E
-
87153-04-6
OPC 13213

-
F
-
87153-06-8
OPC 13217

| Conditions | Yield |
|---|---|
| metabolism studies, pharmacokinetic; |

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
73963-42-5
1-cyclohexyl-5-(4-chlorobutyl)-1,2,3,4-tetrazole

-
-
865792-18-3
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butoxy]-1-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butyl]-3,4-dihydro-1H-quinolin-2-one

| Conditions | Yield |
|---|---|
| With sodium hydride In DMF (N,N-dimethyl-formamide) at 10 - 15℃; for 27.5h; |
-
-
1227960-59-9
4-bromo-3-(bromomethyl)butanoyl chloride

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
1227960-58-8
1-(4-Bromo-3-(bromomethyl)butanoyl)-6-(4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy)-3,4-dihydroquinolin-2(1H)-one

| Conditions | Yield |
|---|---|
| With triethylamine; dmap In tetrahydrofuran at 0 - 20℃; Inert atmosphere; |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
1266615-51-3
CLZ-I2

| Conditions | Yield |
|---|---|
| With iodine In 2-methyl-propan-1-ol at 24.84 - 119.84℃; Kinetics; Solvent; Temperature; |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
84-58-2
2,3-dicyano-5,6-dichloro-p-benzoquinone
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
84-58-2
2,3-dicyano-5,6-dichloro-p-benzoquinone

-
-
1266615-52-4
CLZ-DDQ

| Conditions | Yield |
|---|---|
| In acetonitrile for 298 - 313h; Kinetics; Solvent; Temperature; |
-
-
490-79-9
2,5-dihydroxybenzoic acid.

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
1352614-11-9
cilostazol gentisic acid

| Conditions | Yield |
|---|---|
| In butanone at 20 - 50℃; for 120h; Solvent; Temperature; Time; Concentration; | |
| In acetone at 20 - 40℃; for 168h; |
-
-
490-79-9
2,5-dihydroxybenzoic acid.

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
1352614-12-0
cilostazol gentisic acid monohydrate

| Conditions | Yield |
|---|---|
| With water In nitromethane at 20 - 50℃; for 120h; Time; |
-
-
89-86-1
4-hydroxysalicylic acid

-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
1352614-14-2
cilostazol 2,4-dihydroxybenzoic acid

| Conditions | Yield |
|---|---|
| In nitromethane at 20 - 50℃; for 120h; Solvent; Temperature; Time; Concentration; | |
| In acetone at 20℃; for 168h; |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
99-96-7
4-hydroxy-benzoic acid

-
-
1352614-13-1
cilostazol 4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| In nitromethane at 20 - 50℃; for 120h; Solvent; Temperature; Time; Concentration; | |
| In acetone at 20℃; for 168h; |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

-
-
128446-35-5
heptakis-(2,3,6-tris-(2-hydroxypropyl))-β-cyclodextrin

| Conditions | Yield |
|---|---|
| In ethanol; water at 30℃; |
-
-
73963-72-1
6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydride / mineral oil / 0.5 h / 0 °C / Inert atmosphere 1.2: 12 h / 0 - 20 °C / Inert atmosphere 2.1: 1,1,3,3-Tetramethyldisiloxane; bis(triphenylphosphine)carbonyliridium(I) chloride / dichloromethane / 0.5 h / 20 °C 2.2: 3 h / 20 °C View Scheme |
Cilostazol Specification
1. Introduction of Cilostazol
Cilostazol is one kind of white crystalline powder or off-white solid. The IUPAC Name of it is 6-[4-(1-cyclohexyltetrazol-5-yl)butoxy]-3,4-dihydro-1H-quinolin-2-one. The Solubility of it is DMSO: 18 mg/mL, soluble. Besides, it belongs to Intermediates & Fine Chemicals;Isotope Labeled Compounds;Pharmaceuticals.
Its Classification Code is Anti-Asthmatic Agents; Antithrombotic; Autonomic Agents; Bronchodilator Agents; Cardiovascular Agents; Central Nervous System Agents; Drug / Therapeutic Agent; Enzyme Inhibitors; Fibrin Modulating Agents; Fibrinolytic Agents; Hematologic Agents; Human Data; Inhibitor [platelet]; Neuroprotective Agents; Peripheral Nervous System Agents; Phosphodiesterase 3 Inhibitors; Phosphodiesterase Inhibitors; Platelet Aggregation Inhibitors; Protective Agents; Reproductive Effect; Respiratory System Agents; Vasodilator; Vasodilator Agents.
2. Properties of Cilostazol
Physical properties about Cilostazol are:
(1)Empirical Formula: C20H27N5O2; (2)H bond acceptors: 7; (3)H bond donors: 1; (4)Freely Rotating Bonds: 7; (5)Polar Surface Area: 73.14 Å2; (6)Index of Refraction: 1.675; (7)Molar Refractivity: 102.93 cm3; (8)Molar Volume: 273.7 cm3; (9)Surface Tension: 54.9 dyne/cm; (10)Density: 1.34 g/cm3; (11)Flash Point: 355.8 °C; (12)Enthalpy of Vaporization: 97.74 kJ/mol; (13)Boiling Point: 664.7 °C at 760 mmHg; (14)Vapour Pressure: 1.56E-17 mmHg at 25°C; (15)Melting point: 159-160°C.
3. Structure Descriptors of Cilostazol
(1)InChI: InChI=1/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
(2)Smiles: n1(c(nnn1)CCCCOc1cc2c(NC(=O)CC2)cc1)C1CCCCC1
(3)InChIKey: InChIKey=RRGUKTPIGVIEKM-UHFFFAOYSA-N
4. Toxicity of Cilostazol
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | > 2gm/kg (2000mg/kg) | Drugs in Japan Vol. -, Pg. 504, 1990. | |
| man | TDLo | oral | 1248ug/kg (1.248mg/kg) | BEHAVIORAL: HEADACHE | Arzneimittel-Forschung. Drug Research. Vol. 35, Pg. 1173, 1985. |
| mouse | LD50 | intramuscular | > 1gm/kg (1000mg/kg) | Drugs in Japan Vol. -, Pg. 504, 1990. | |
| mouse | LD50 | intraperitoneal | > 2gm/kg (2000mg/kg) | Drugs in Japan Vol. -, Pg. 504, 1990. | |
| mouse | LD50 | oral | > 5gm/kg (5000mg/kg) | Drugs in Japan Vol. -, Pg. 504, 1990. | |
| rat | LD50 | intramuscular | > 1gm/kg (1000mg/kg) | Drugs in Japan Vol. -, Pg. 504, 1990. | |
| rat | LD50 | intraperitoneal | > 2gm/kg (2000mg/kg) | Drugs in Japan Vol. -, Pg. 504, 1990. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Drugs in Japan Vol. -, Pg. 504, 1990. |
5. Safety information of Cilostazol
Possible side effects of cilostazole use include headache (the most common), diarrhea, abnormal stools, increased heart rate, and palpitations. Although drugs similar to cilostazol have increased the risk of death in patients with congestive heart failure, studies of significant size have not addressed people without the disease.
Hazard Codes:
 Xi
XiWGK Germany: 2
RTECS: VC8277500
6. Production of Cilostazol
Cilostazole is manufactured by Otsuka Pharmaceutical Co. under the trade name Pletal. 5-chloro-N-cyclohexyl amyl amide of benzene solution can used to manufacture Cilostazole. But it will take place under the condition of Phosphorus pentachloride, benzene, ammonia and potassium hydroxide solution. All is showed as follows:
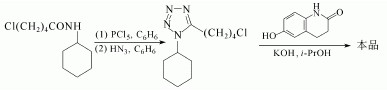
7. Uses of Cilostazol
Cilostazol (CAS NO.73963-72-1) is a medication used in the alleviation of the symptom of intermittent claudication in individuals with peripheral vascular disease. Cilostazol is a potent phosphodiesterase III A (PDE3A) inhibitor (IC50=0.2uM) and inhibitor of adenosine uptake. In addition, it has antimitogeni, antithrombotic, vasodilatory and cardiotonic properties in vivo. It can also affects lipid levels in vivo. Besides, it can also used in the clinical use. Cilostazol is a phosphodiesterase inhibitor with therapeutic focus on cAMP. It inhibits platelet aggregation and is a direct arterial vasodilator. Its main effects are dilation of the arteries supplying blood to the legs and decreasing platelet coagulation.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View