-
Name
Cinchonidine
- EINECS 207-622-3
- CAS No. 485-71-2
- Article Data21
- CAS DataBase
- Density 1.204 g/cm3
- Solubility insoluble in water
- Melting Point 204-206 °C(lit.)
- Formula C19H22N2O
- Boiling Point 464.502 °C at 760 mmHg
- Molecular Weight 294.396
- Flash Point 234.723 °C
- Transport Information UN 1544
- Appearance white to light yellow crystal powder
- Safety 22-24/25-36/37-26
- Risk Codes 20/21/22-42/43-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Cinchonan-9-ol, (8alpha,9R)-;(R)-[(4R,5S,7R)-5-ethenyl-1-azoniabicyclo[2.2.2]oct-7-yl]-quinolin-4-yl-methanol;(R)-[(5S,7S)-5-ethenyl-1-azabicyclo[2.2.2]oct-7-yl]-quinolin-4-yl-methanol;(R)-[(4S,5S,7R)-5-ethenyl-1-azoniabicyclo[2.2.2]oct-7-yl]-quinolin-4-yl-methanol;Cinchonan-9-ol, (8R,9R)-;Cinchonan-9-ol, (8-alpha,9R)- (9CI);2-Quinuclidinemethanol, alpha-4-quinolyl-5-vinyl-;(R)-[(4S,5S,7S)-5-ethenyl-1-azabicyclo[2.2.2]oct-7-yl]-quinolin-4-yl-methanol;(8S,9R)-Cinchonidine;alpha-Quinidine;(8-alpha,9R)-Cinchonan-9-ol;
- PSA 36.36000
- LogP 3.10250
Synthetic route

-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| With potassium 4-(methoxy)phenyltrifluoroborate In dimethyl sulfoxide at 37℃; for 10h; Inert atmosphere; | 64% |
-
-
915134-81-5
O-tosylcinchonidine

-
A
-
1393446-38-2
(Z)-1-(quinolin-4-yl)-3-((3R,4R)-3-vinylpiperidin-4-yl)prop-1-enyl 4-methylbenzene sulfonate

-
B
-
118-10-5, 485-70-1, 485-71-2, 550-54-9, 40134-63-2, 72402-55-2, 72402-56-3
(1S,3R,4S,8S,9S)-9-hydroxy-cinchonane

-
C
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| With salicylic acid In ethylene glycol at 95℃; for 0.25h; Microwave irradiation; | A 40% B 20% C 10% |


-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| ueber mehrere Stufen; |
-
-
71-41-0
pentan-1-ol

-
-
118-10-5
Cinchonin

-
A
-
118-10-5, 485-70-1, 485-71-2, 550-54-9, 40134-63-2, 72402-55-2, 72402-56-3
(1S,3R,4S,8S,9S)-9-hydroxy-cinchonane

-
B
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| Product distribution; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide; pentan-1-ol |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -10 - 20℃; for 5h; Inert atmosphere; | 100% |
| In tetrahydrofuran at -10 - 50℃; for 6h; Inert atmosphere; Schlenk technique; | 100% |
| In tetrahydrofuran at -78.16℃; for 12h; Inert atmosphere; | 14% |
| In tetrahydrofuran at -78.16℃; for 12h; Inert atmosphere; | 14% |
-
-
100-44-7
benzyl chloride

-
-
485-71-2
Cinchonidin

-
-
69221-14-3, 69257-04-1, 95189-65-4
(8S,9R)-(-)-N-benzylcinchonidinium chloride

| Conditions | Yield |
|---|---|
| In toluene for 2h; Reflux; | 100% |
| With sodium iodide In tetrahydrofuran at 20℃; Reflux; |
-
-
23165-29-9
3,5-bistrifluoromethylphenylisothiocyanate

-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 12h; Inert atmosphere; | 99% |
-
-
215169-00-9
2-{3-[(1R)-3-(3,4-dimethoxyphenyl)-1-hydroxypropyl]phenoxy}acetic acid

-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| In ethyl acetate at 50℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: Cinchonidin With potassium hydride In N,N-dimethyl-formamide; mineral oil; pentane at 0 - 50℃; for 3h; Inert atmosphere; Stage #2: methyl iodide In N,N-dimethyl-formamide; mineral oil; pentane at 0 - 20℃; Inert atmosphere; | 98% |
| With sodium hydride In N,N-dimethyl-formamide at 0 - 50℃; for 2.5h; | 46% |
| Stage #1: Cinchonidin With sodium hydride In tetrahydrofuran; mineral oil at 0 - 50℃; for 1.5h; Inert atmosphere; Schlenk technique; Stage #2: methyl iodide In tetrahydrofuran; mineral oil at 0℃; for 72h; Inert atmosphere; Schlenk technique; | 1% |
-
-
96574-31-1
N2-acetyl-2,O4-dimethyltyrosine

-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| 97.4% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
485-71-2
Cinchonidin

-
-
183994-23-2
4-[(R)-(tert-Butyl-dimethyl-silanyloxy)-((1S,2S,4S,5R)-5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl)-methyl]-quinoline

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide for 24h; Ambient temperature; | 97% |
-
-
485-71-2
Cinchonidin

-
-
415679-05-9
4-(bromomethyl)-N-tert-butylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| In methanol at 40℃; for 15h; | 97% |

| Conditions | Yield |
|---|---|
| In methanol at 40℃; for 15h; | 97% |

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 20h; | 97% |

| Conditions | Yield |
|---|---|
| In ethanol; chloroform; N,N-dimethyl-formamide at 100℃; for 6h; | 96% |
| In ethanol; chloroform; N,N-dimethyl-formamide at 100℃; for 4h; | 72% |
| In ethanol; chloroform; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| In ethanol; chloroform; N,N-dimethyl-formamide at 100℃; for 6h; | 96% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 12h; Inert atmosphere; Reflux; | 96% |
-
-
100-11-8
1-bromomethyl-4-nitro-benzene

-
-
485-71-2
Cinchonidin

-
-
123355-59-9
(2S,4S,5R)-2-((R)-hydroxy(quinolin-4-yl)methyl)-1-(4-nitrobenzyl)-5-vinylquinuclidin-1-ium bromide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Reflux; | 95% |
| In acetonitrile Inert atmosphere; | 83% |
| In tetrahydrofuran for 4h; Heating; |

| Conditions | Yield |
|---|---|
| In acetonitrile Inert atmosphere; | 95% |
| In tetrahydrofuran Reflux; | 80% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 3h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 20h; | 94% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 20h; | 94% |

| Conditions | Yield |
|---|---|
| In methanol at 40℃; for 18h; | 94% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 20h; | 94% |
-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| With bromine; triethylamine In chloroform at 0 - 20℃; for 4h; | 94% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Reflux; | 93% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; for 10h; | 92% |
| With hydrogen; palladium on activated charcoal In methanol at 20℃; for 12h; | 86% |
| With triethylsilane; 1% Pd on activated carbon In water at 45℃; for 24h; Reagent/catalyst; Green chemistry; chemoselective reaction; | 83% |
-
-
861815-63-6
4-(bromomethyl)phenyl[di(1-naphthyl)]methanol

-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| In toluene; acetonitrile at 80℃; for 6h; | 92% |
-
-
1308718-76-4
4-(bromomethyl)-N-(phenyl)benzenesulfonamide

-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 20h; | 92% |

-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| In ethanol; chloroform; N,N-dimethyl-formamide at 100℃; for 4h; | 91% |
| In tetrahydrofuran for 4h; Heating; |
-
-
93629-15-3
5-(bromomethyl)-1,3-di-tert-butyl-2-methoxybenzene

-
-
485-71-2
Cinchonidin

| Conditions | Yield |
|---|---|
| In toluene for 4h; Heating; | 91% |

| Conditions | Yield |
|---|---|
| In methanol at 40℃; for 15h; | 90% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Reflux; | 90% |
Cinchonidine Consensus Reports
Cinchonidine Specification
The Cinchonidine, with the CAS registry number 485-71-2, is also known as (8S,9R)-Cinchonidine. It belongs to the product categories of Cyclic compounds; Alkaloids; Chiral; Biochemistry; Optical Resolution; Quinoline Alkaloids; Quinoline carboxylic Acids, etc.; Quinolines; Synthetic Organic Chemistry; Aromatics; Chiral Reagents; Heterocycles; Intermediates & Fine Chemicals; Pharmaceuticals. Its EINECS number is 207-622-3. This chemical's molecular formula is C19H22N2O and molecular weight is 294.39. What's more, its systematic name is (8α,9R)-Cinchonan-9-ol. Its classification codes are: (1)Drug / Therapeutic Agent; (2)Natural Product. This chemical is an alkaloid found in Cinchona officinalis. It is used in asymmetric synthesis in organic chemistry. It is a stereoisomer and pseudo-enantiomer of cinchonine. This chemcial should be sealed and stored in a ventilated and dry place. Moreover, it should be protected from oxides.
Physical properties of Cinchonidine are: (1)ACD/LogP: 2.788; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.29; (4)ACD/LogD (pH 7.4): 0.90; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 10.06; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 36.36 Å2; (13)Index of Refraction: 1.652; (14)Molar Refractivity: 89.424 cm3; (15)Molar Volume: 244.537 cm3; (16)Polarizability: 35.45×10-24cm3; (17)Surface Tension: 56.4 dyne/cm; (18)Density: 1.204 g/cm3; (19)Flash Point: 234.723 °C; (20)Enthalpy of Vaporization: 76.485 kJ/mol; (21)Boiling Point: 464.502 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
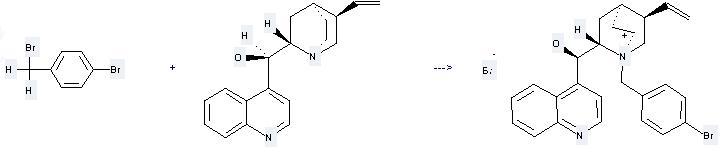
Uses of Cinchonidine: it can be used to produce N-(p-bromobenzyl)-cinchonidinium bromide at the ambient temperature. It will need solvent CH2Cl2 with the reaction time of 17 hours. The yield is about 65%.
When you are using this chemical, please be cautious about it as the following:
This chemcial is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. This substance may cause sensitisation by inhalation and skin contact. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. You should not breathe dust. When using it, you need wear suitable protective clothing and gloves and you must avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: n2c1c(cccc1)c(cc2)[C@@H](O)[C@H]3N4CC[C@@H](C3)[C@@H](/C=C)C4
(2)Std. InChI: InChI=1S/C19H22N2O/c1-2-13-12-21-10-8-14(13)11-18(21)19(22)16-7-9-20-17-6-4-3-5-15(16)17/h2-7,9,13-14,18-19,22H,1,8,10-12H2/t13-,14-,18-,19+/m0/s1
(3)Std. InChIKey: KMPWYEUPVWOPIM-KODHJQJWSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| quail | LD50 | oral | > 316mg/kg (316mg/kg) | Ecotoxicology and Environmental Safety. Vol. 6, Pg. 149, 1982. | |
| rat | LD50 | intraperitoneal | 206mg/kg (206mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | Acta Pharmacologica et Toxicologica. Vol. 4, Pg. 265, 1948. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View