-
Name
6-Octen-1-ol,3,7-dimethyl-
- EINECS 203-375-0
- CAS No. 106-22-9
- Article Data270
- CAS DataBase
- Density 0.845 g/cm3
- Solubility slightly soluble in water
- Melting Point 77-83 °C(lit.)
- Formula C10H20O
- Boiling Point 224.499 °C at 760 mmHg
- Molecular Weight 156.268
- Flash Point 98.333 °C
- Transport Information
- Appearance colourless liquid
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,3-Dihydrogeraniol;2,6-Dimethyl-2-octen-8-ol;3,7-Dimethyl-6-octen-1-ol;Cephrol;Citronellol 950;Dihydrogeraniol;Rodinol;
- PSA 20.23000
- LogP 2.75130
Synthetic route
-
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With Zn(BH4)2(Ph3P)2 In tetrahydrofuran at 20℃; Reduction; | 100% |
| With hydrogen; aluminum oxide; copper In isopropyl alcohol at 90℃; for 0.75h; | 100% |
| With trimethylamine-N-oxide; sodium formate; C34H44FeN4O4(2+)*2I(1-) In water at 80℃; for 24h; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogen; aluminum oxide; copper In isopropyl alcohol at 90℃; for 12h; | 100% |
| With hydrogen; polymer-supported rhodium catalyst In dichloromethane under 1551.49 Torr; for 10h; Hydrogenation; | 96% |
| With 1,1'-bis(diphenylphosphino)ferrocene; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; Butane-1,4-diol; potassium tert-butylate at 110℃; for 24h; Inert atmosphere; | 71% |

| Conditions | Yield |
|---|---|
| With hydrogen; aluminum oxide; copper In isopropyl alcohol at 90℃; for 12h; | 100% |
| With dichloro(η3:η2:η3-dodeca-2,6,10-triene-1,12-diyl)ruthenium(IV); caesium carbonate; isopropyl alcohol at 82℃; for 23h; Inert atmosphere; chemoselective reaction; | 95 %Chromat. |
-
-
96154-40-4
1-(benzyloxy)-3,7-dimethyloct-6-ene

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With hydrogen; benzyl bromide; Pd<*>MCM-48 In methanol under 760.051 Torr; for 0.5h; | 99% |
| With aluminium trichloride; N,N-dimethyl-aniline In dichloromethane for 1.5h; Ambient temperature; | 98% |
| With naphthalene; lithium In tetrahydrofuran; methanol at -78℃; for 40h; | 89% |

| Conditions | Yield |
|---|---|
| With [RuCl2((E)-N-(2-(diphenylphosphino)benzyl)-1-(6-((diphenylphosphino)methyl)pyridin-2-yl)methanimine)]; hydrogen; sodium ethanolate at 80℃; under 37503.8 Torr; for 5h; Autoclave; | 98% |
| With lithium aluminium tetrahydride Yield given; |

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In water; acetonitrile at 0℃; for 0.5h; Product distribution; | 98% |
-
-
90243-41-7
2-(3,7-dimethyloct-6-enyloxy)-tetrahydro-2H-pyran

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With SA-3 silica-alumina gel In methanol at 120℃; for 2.16667h; | 98% |
| With SA-3 silica-alumina gel In methanol at 120℃; for 2.16667h; Product distribution; other ethers, var. silica-alumina gels, temp. and time; | 98% |
| With copper dichloride In methanol at 20℃; Hydrolysis; | 90% |
| With CuCl2*2H2O In methanol at 20℃; for 1.25h; | 90% |
| With boron trifluoride diethyl etherate; sodium cyanoborohydride In tetrahydrofuran for 3h; Ambient temperature; | 80% |

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With SA-3 silica-alumina gel In methanol at 120℃; for 1.33333h; | 98% |

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With dibromotriphenylphosphorane In dichloromethane at -50℃; for 2h; | 97% |
-
-
18419-09-5
(3,7-dimethyloct-6-enyloxy)trimethylsilane

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With SA-3 silica-alumina gel In methanol for 1.16667h; Ambient temperature; | 97% |
| With 18-crown-6 ether In ethanol; water at 45℃; for 3h; Green chemistry; | 91% |

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With SA-2 silica-alumina gel In methanol for 3.16667h; Ambient temperature; | 97% |
-
-
106-26-3
cis-3,7-dimethyl-2,6-octadienal

-
A
-
106-22-9
Citronellol

-
B
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

| Conditions | Yield |
|---|---|
| With acetylacetonato(1,5-cyclooctadiene)rhodium(I); hydrogen; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 25℃; under 750.075 Torr; for 0.5h; Catalytic behavior; Temperature; Pressure; Autoclave; chemoselective reaction; | A 3% B 97% |
| With acetylacetonatodicarbonylrhodium(l); hydrogen; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In neat (no solvent) at 60℃; under 30003 Torr; for 10h; Temperature; Pressure; Autoclave; | A 88 %Spectr. B 12 %Spectr. |

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With cerium(III) chloride In acetonitrile for 1h; Heating; | 96% |

| Conditions | Yield |
|---|---|
| With LaNi5 hydride In tetrahydrofuran; methanol for 12h; Ambient temperature; | 95% |
| With LaNi5 hydride In tetrahydrofuran; methanol 1) 0 deg C, 6 h, 2) r.t., 12 h; | 95% |
| With hydrido(triphenylphosphine)copper(I) hexamer In tetrahydrofuran for 24h; Ambient temperature; | 90% |
-
-
5392-40-5
(E/Z)-3,7-dimethyl-2,6-octadienal

-
A
-
106-22-9
Citronellol

-
B
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

| Conditions | Yield |
|---|---|
| With hydrogen; Ni0.88Cr0.12 In cyclohexane at 79.9℃; under 7575.6 Torr; Product distribution; other catalysts, hydrogenation rates, selectivity of catalysts; | A n/a B 92% |
| With hydrogen; Ni-Gr2 In methanol at 45℃; under 22800 Torr; for 18h; | A 68% B 26% |
| With hydrogen; sodium carbonate; chromium(III) oxide; nickel In water; isopropyl alcohol at 120℃; under 30400 Torr; Product distribution; other temperature, other solvent, other pressure, other catalysts; | A 0.6 % Chromat. B 97.5 % Chromat. |
-
-
87921-26-4
tert-butyl((3,7-dimethyloct-6-en-1-yl)oxy)dimethylsilane

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With 2,5-bis(perfluorobutyl)-1,4-benzoquinone In water; acetonitrile at 20℃; for 6h; | 92% |
| With oxone In methanol at 20℃; for 15h; | 80% |
| With bismuth(III) chloride; sodium iodide In acetonitrile at 20℃; for 1.5h; | 76% |
-
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

-
-
62-53-3
aniline

-
A
-
106-22-9
Citronellol

-
B
-
31043-21-7
N-(3,7-dimethyloct-6-en-1-yl)aniline

| Conditions | Yield |
|---|---|
| Stage #1: 3,7-dimethyl-oct-6-enal; aniline at 25℃; for 0.166667h; Stage #2: With sodium tetrahydroborate; boric acid at 25℃; for 0.333333h; | A n/a B 92% |


-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane desilylation; Photolysis; | 91% |

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether In ethanol; water at 45℃; for 2.5h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Green chemistry; | 91% |

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at -20℃; | 90% |

| Conditions | Yield |
|---|---|
| With Ba(BH2S3)2 In tetrahydrofuran at 20℃; for 2.5h; | 90% |
| With hydrogen In isopropyl alcohol at 70℃; under 56255.6 Torr; for 2h; Reagent/catalyst; Autoclave; Glovebox; |
-
-
5392-40-5
(E/Z)-3,7-dimethyl-2,6-octadienal

-
A
-
106-22-9
Citronellol

-
B
-
106-21-8, 59204-02-3
tetrahydrogeraniol

-
-
15356-70-4
menthol

| Conditions | Yield |
|---|---|
| With hydrogen In tert-butyl alcohol at 80℃; under 1500.15 - 15001.5 Torr; for 8h; Autoclave; diastereoselective reaction; | A n/a B n/a C 89% |


| Conditions | Yield |
|---|---|
| With hydrogen; silver In tetrahydrofuran at 150℃; under 11251.1 Torr; for 72h; | A 10% B 88% |
| With sodium cyanoborohydride at 20℃; for 0.05h; | A 46% B 54% |
| With (triphenylphosphine)copper(I) hydride hexamer; hydrogen; Dimethyl(phenyl)phosphine In tert-butyl alcohol; benzene at 20℃; under 25857.4 Torr; for 15h; Hydrogenation; Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| With chlorine[2-(4,5-dihydro-1H-imidazol-2-yl)-6-methoxypyridine](pentamethylcyclopentadienyl)iridium(III) chloride; sodium formate In water at 80℃; for 0.5h; Schlenk technique; chemoselective reaction; | A 88% B n/a |

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With samarium diiodide; water; isopropylamine In tetrahydrofuran at 20℃; for 0.0833333h; | 87% |
| With naphthalene; lithium In tetrahydrofuran; methanol at -78℃; for 40h; | 86% |
-
-
5392-40-5
(E/Z)-3,7-dimethyl-2,6-octadienal

-
A
-
106-22-9
Citronellol

-
B
-
106-21-8, 59204-02-3
tetrahydrogeraniol

-
-
15356-70-4
menthol

| Conditions | Yield |
|---|---|
| With hydrogen In tert-butyl alcohol at 80℃; under 7500.75 Torr; for 8h; Autoclave; diastereoselective reaction; | A n/a B n/a C 87% D n/a |

-
-
41144-01-8
(+/-)-citronellyl tosylate

-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With tetraethylammonium perchlorate; triethylamine In dimethyl sulfoxide at 20℃; for 10h; Electrolysis; Green chemistry; | 87% |
-
-
110109-86-9
3,7-dimethyl-1-acetoxy-6-p-toluenesulfonyloxy octane

-
A
-
106-22-9
Citronellol

-
B
-
106-21-8, 59204-02-3
tetrahydrogeraniol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran 1.) -10 deg C 2.) RT; | A 6% B 82% |
-
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

-
A
-
106-22-9
Citronellol

-
B
-
13887-74-6, 90544-01-7
(3,7-dimethyloctyl)amine

-
C
-
53339-59-6
3,7-dimethyl-6-octenylamine

-
D
-
24381-83-7
3,3',7,7'-tetramethyldi-6-octenylamine

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; hydrogen; ammonium hydroxide; 1-methyl-3-decylimidazolium bromide In toluene at 130℃; under 45004.5 Torr; for 6h; Reagent/catalyst; Autoclave; | A 8% B 1% C 81% D n/a E n/a F 8% |
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; hydrogen; ammonium hydroxide; 1-octyl-3-methyl-imidazolium bromide In toluene at 130℃; under 45004.5 Torr; for 6h; Autoclave; | A 20% B 2% C 41% D n/a E n/a F 21% |


-
-
106-22-9
Citronellol

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium azide; trisodium tris(3-sulfophenyl)phosphine In water; acetonitrile at 25℃; for 1h; | 80% |

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In dichloromethane at 0℃; for 2h; | 100% |
| With 1H-imidazole; iodine In dichloromethane at 20℃; for 0.75h; | 93% |
| With pyridine; bromine; triphenylphosphine In tetrachloromethane; N,N-dimethyl-formamide at 45℃; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: Citronellol With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Stage #2: benzyl bromide With sodium iodide In tetrahydrofuran for 5h; Heating; | 100% |
| Stage #1: Citronellol With sodium hydride In tetrahydrofuran; hexane for 2h; Heating; Stage #2: benzyl bromide With tetra-(n-butyl)ammonium iodide In tetrahydrofuran; hexane for 18h; Heating; Further stages.; | 58% |
| With sodium hydride 1.) dimethoxyethane, room temperature, 2 h, 2.) room temperature, 40 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 5℃; for 1h; | 100% |
| With potassium hydroxide; potassium carbonate for 0.05h; | 93% |
| With pyridine In chloroform for 0.5h; sonication; | 92% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; | 100% |
| With C12H8N2*2CH4O3S at 60℃; for 3h; | 99% |
| With iodine at 25℃; for 0.0166667h; | 98% |
-
-
106-22-9
Citronellol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
87921-26-4
tert-butyl((3,7-dimethyloct-6-en-1-yl)oxy)dimethylsilane

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 24h; | 100% |
| With dmap; triethylamine | 96% |
| With 1H-imidazole In dichloromethane at 0 - 20℃; for 16h; | 90% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 5h; Inert atmosphere; | 100% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 22h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B at 50℃; for 4h; Molecular sieve; Ionic liquid; Green chemistry; Enzymatic reaction; | 99.9% |
| In n-heptane at 40℃; for 24h; lipozyme IM 20 (immobilized Mucor miehi lipase); | 94.66% |
| In water at 30℃; for 18h; lipase from Aspergillus niger; Yield given; |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B at 50℃; for 4h; Molecular sieve; Ionic liquid; Green chemistry; Enzymatic reaction; | 99.9% |
| In water at 30℃; for 18h; lipase from Aspergillus niger; Yield given; |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B at 50℃; for 4h; Molecular sieve; Ionic liquid; Green chemistry; Enzymatic reaction; | 99.9% |
| In water at 30℃; for 18h; lipase from Aspergillus niger; Yield given; |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B at 50℃; for 4h; Molecular sieve; Ionic liquid; Green chemistry; Enzymatic reaction; | 99.9% |
| With cetyltrimethylammonium chloride; potassium hexacyanoferrate(III) at 80℃; for 1h; | 90% |
| With molecular sieve at 120℃; for 8h; |

| Conditions | Yield |
|---|---|
| With triethylamine at 20℃; | 99.5% |
| With triethylamine In dichloromethane at 0℃; for 3h; Inert atmosphere; | 64% |
-
-
106-22-9
Citronellol

-
-
106-21-8, 59204-02-3
tetrahydrogeraniol

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In ethyl acetate | 99% |
| With hydrogen; palladium on activated charcoal In ethyl acetate at 80℃; under 2585.81 Torr; for 0.0833333h; microwave irradiation; | 99% |
| With hydrogen; palladium on activated charcoal In methanol at 20℃; for 50h; | 93% |
-
-
106-22-9
Citronellol

-
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

| Conditions | Yield |
|---|---|
| With pyridine chromium peroxide In dichloromethane for 1.25h; Product distribution; Ambient temperature; effect of various chromium(VI) based oxidants; | 99% |
| With pyridine chromium peroxide In dichloromethane for 1.25h; Ambient temperature; | 99% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 3-[4'-(diacetoxyiodo)phenoxy]-1-propyl-N,N,N-trimethylammonium 4-methylbenzenesulfonate In dichloromethane at 20℃; for 2h; | 99% |
-
-
106-22-9
Citronellol

-
-
124-63-0
methanesulfonyl chloride

-
-
42602-37-9
(±)-3,7-dimethyloct-6-en-1-yl methanesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 3h; Inert atmosphere; | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; for 24.3h; | 97% |
| With triethylamine In dichloromethane at 0 - 20℃; for 16h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane Epoxidation; | 98% |
| With oxygen; cobalt(II) acetate; isobutyraldehyde In dichloromethane at 20℃; for 5h; | 98% |
| With (NMe4)(Co-ortho-phenylenebis(N'-methyloxamidate)*2H2O*CH3CN; oxygen; pivalaldehyde In fluorobenzene for 2.5h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| With potassium fluoride on basic alumina In toluene for 1h; | 98% |
-
-
106-22-9
Citronellol

-
-
74-95-3
1,2-dibromomethane

-
-
321858-86-0
5-(2,2-dimethylcyclopropyl)-3-methylpentan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: Citronellol; 1,2-dibromomethane With triisobutylaluminum at 10 - 20℃; for 0.25h; Stage #2: With iron(III) chloride; triisobutylaluminum at 25℃; for 3h; regioselective reaction; | 98% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
106-22-9
Citronellol

-
-
18419-09-5
(3,7-dimethyloct-6-enyloxy)trimethylsilane

| Conditions | Yield |
|---|---|
| With 1,1,1,3,3,3-hexamethyl-disilazane at 25℃; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; Inert atmosphere; | 98% |
Citronellol Consensus Reports
Citronellol (106-22-9) is reported in EPA TSCA Inventory.
Citronellol Specification
The Citronellol, with the CAS registry number 106-22-9, is also known as 2,3-Dihydrogeraniol. It belongs to the product categories of Acyclic Monoterpenes; Biochemistry; Terpenes; Alphabetical Listings; C-D; Flavors and Fragrances; Acyclic; Alcohols; Alkenes; Building Blocks; C9 to C10; Chemical Synthesis; Organic Building Blocks; Oxygen Compounds. Its EINECS registry number is 203-375-0. This chemical's molecular formula is C10H20O and molecular weight is 156.27. What's more, both its IUPAC name and systematic name are the same which is called 3,7-Dimethyloct-6-en-1-ol. It should be stored in a cool, dry and well-ventilated place.
Physical properties about Citronellol are: (1)ACD/LogP: 3.382; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.38; (4)ACD/LogD (pH 7.4): 3.38; (5)ACD/BCF (pH 5.5): 218.92; (6)ACD/BCF (pH 7.4): 218.92; (7)ACD/KOC (pH 5.5): 1647.35; (8)ACD/KOC (pH 7.4): 1647.35; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 6; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.45; (14)Molar Refractivity: 49.772 cm3; (15)Molar Volume: 184.902 cm3; (16)Polarizability: 19.731×10-24cm3; (17)Surface Tension: 28.578 dyne/cm; (18)Density: 0.845 g/cm3; (19)Flash Point: 98.333 °C; (20)Enthalpy of Vaporization: 53.601 kJ/mol; (21)Boiling Point: 224.499 °C at 760 mmHg; (22)Vapour Pressure: 0.018 mmHg at 25 °C.
Preparation of Citronellol: this chemical can be prepared by 8-allyloxy-2,6-dimethyl-oct-2-ene. This reaction needs reagents Li, ROMP gel supported naphthalene and solvents tetrahydrofuran, methanol at temperature of -78 °C. The reaction time is 40 hours. The yield is 86 %.

Uses of Citronellol: (1) it is can be used for the production of paint desiccant, dyeing mordant, glass and steel curing accelerator, and invisible ink and so on; (2) it is used to produce other chemicals. For example, it can react with propionic acid methyl ester to get (S)-citronellyl propionate. The reaction occurs with reagent 0.1 M phosphate buffer and other condition of ambient temperature for 96 hours. The yield is 58 %.
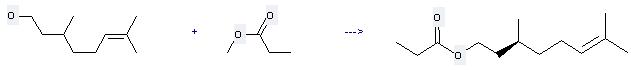
When you are dealing with this chemical, you should be very careful. This chemical may cause inflammation to the skin or other mucous membranes. And it is irritating to eyes, respiratory system and skin. Therefore, you should wear suitable protective clothing and avoid contacting with skin and eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: C\C(C)=C\CCC(C)CCO
(2) InChI: InChI=1S/C10H20O/c1-9(2)5-4-6-10(3)7-8-11/h5,10-11H,4,6-8H2,1-3H3
(3) InChIKey: QMVPMAAFGQKVCJ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intramuscular | 4gm/kg (4000mg/kg) | Journal of Scientific and Industrial Research, Section C: Biological Sciences. Vol. 21, Pg. 342, 1962. | |
| mouse | LD50 | subcutaneous | 880mg/kg (880mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE | Sapporo Igaku Zasshi. Sapporo Medical Journal. Vol. 3, Pg. 73, 1952. |
| rabbit | LD50 | skin | 2650mg/kg (2650mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 757, 1975. | |
| rat | LD50 | oral | 3450mg/kg (3450mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 757, 1975. |
Related Products
- Citronellol
- Citronellolformate
- 106229-05-4
- 106-23-0
- 106232-85-3
- 106232-86-4
- 1062368-24-4
- 1062368-70-0
- 1062368-71-1
- 106-24-1
- 106243-16-7
- 106243-23-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View