-
Name
Cyclamic acid
- EINECS 202-898-1
- CAS No. 100-88-9
- Article Data21
- CAS DataBase
- Density 1.32g/cm3
- Solubility Soluble SOLVENT
- Melting Point 165 - 170 C (Decomposes)
- Formula C6H13 N O3 S
- Boiling Point °Cat760mmHg
- Molecular Weight 179.24
- Flash Point °C
- Transport Information 25kgs
- Appearance white crystals
- Safety Suspected human carcinogen producing bladder tumors. Poison by intravenous route. Mildly toxic by ingestion. When heated to decomposition it emits toxic fumes of SOx and NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols UN NO.
- Synonyms Cyclohexanesulfamicacid (6CI,7CI,8CI);Sulfamic acid, cyclohexyl- (9CI);Cyclamate;Cyclamic acid;Cyclohexylamidosulfuric acid;Cyclohexylaminesulfonic acid;Cyclohexylsulfamicacid;Hexamic acid;N-Cyclohexylsulfamic acid;NSC 220327;Polycat 200;
- PSA 74.78000
- LogP 2.18320
Synthetic route

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In chloroform for 8h; Ambient temperature; | 72% |
| With chlorosulphuric acid; chloroform | |
| With chlorosulfonic acid | |
| With chlorosulfonic acid In acetonitrile at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; sulfur trioxide |

| Conditions | Yield |
|---|---|
| With sulfur dioxide; benzene |

| Conditions | Yield |
|---|---|
| With sodium dithionite; water; sodium phosphate |
-
-
100-88-9
cyclohexylsulfamic acid

-
-
1620401-56-0
(R)-6-methylamino-2-methylheptene

| Conditions | Yield |
|---|---|
| In ethanol at -20℃; Solvent; Temperature; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 95.6% |
-
-
100-88-9
cyclohexylsulfamic acid

-
-
122883-93-6, 199191-69-0
ziprazidone

| Conditions | Yield |
|---|---|
| In ethanol; water at -15 - 80℃; | 95.2% |
-
-
706779-91-1
N-(1-methylpiperidin-4-yl)-N-(4-fluorophenylmethyl)-N′-(4-(2-methylpropyloxyl)phenylmethyl)carbamide

-
-
100-88-9
cyclohexylsulfamic acid

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20 - 42℃; for 5h; | 93.7% |
-
-
100-88-9
cyclohexylsulfamic acid

-
-
302962-49-8
dasatanib

| Conditions | Yield |
|---|---|
| In methanol at 70℃; | 93.4% |
-
-
100-88-9
cyclohexylsulfamic acid

-
-
10314-35-9
cyclohexylsulfamoyl chloride

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In benzene | 91% |
| With phosphorus pentachloride In benzene for 4h; Heating / reflux; | 91% |
| With phosphorus pentachloride | 75% |
-
-
100-88-9
cyclohexylsulfamic acid

-
-
88150-42-9
2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine

-
-
745013-02-9
amlodipine cyclamate

| Conditions | Yield |
|---|---|
| In ethanol; ethyl acetate at 20℃; for 4h; | 87% |
| In ethanol; isopropyl alcohol at 0℃; for 4h; | 86% |
| In methanol; acetonitrile at 20℃; for 4h; | 85% |
| In isopropyl alcohol at 0℃; for 4h; | 82% |
-
-
268203-93-6
5-[2-propyloxy-5-(2-(1-methylpyrrolidin-2-yl)ethylaminosulphonyl)phenyl]-1-methyl-3-propyl-6,7-dihydro-1H-pyrazolo(4,3-d)pyrimidin-7-one

-
-
100-88-9
cyclohexylsulfamic acid

-
-
1240622-32-5
udenafil cyclamate

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; | 83.7% |
-
-
100-88-9
cyclohexylsulfamic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5-(4-(tert-butyl)phenyl)-5H-dibenzo[b,d]thiophen-5-ium bromide With silver(l) oxide In methanol Stage #2: cyclohexylsulfamic acid In methanol at 20℃; | 80% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80℃; for 0.333333h; | A 77% B n/a |

-
-
100-88-9
cyclohexylsulfamic acid


| Conditions | Yield |
|---|---|
| In ethanol at 90℃; for 3h; | 75% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80℃; for 2.5h; | A 68.6% B n/a |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -80℃; Inert atmosphere; | A 67.4% B n/a |


| Conditions | Yield |
|---|---|
| In water for 120h; Sonication; | 63% |
-
-
100-88-9
cyclohexylsulfamic acid

-
-
150322-43-3
prasugel

-
-
1306607-31-7
5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate cyclamate

| Conditions | Yield |
|---|---|
| In acetone at -10℃; for 12h; | 45% |
| In acetone | 45% |
-
-
100-88-9
cyclohexylsulfamic acid

-
-
138337-16-3
potassium N-nitroso-N-cyclohexylsulfamate

| Conditions | Yield |
|---|---|
| With potassium nitrite In water at 4℃; for 0.333333h; | 44% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80℃; for 0.333333h; | A 26% B 30% C 44% |

-
-
1187594-09-7
Baricitinib

-
-
100-88-9
cyclohexylsulfamic acid

| Conditions | Yield |
|---|---|
| In acetone for 0.5h; Reflux; | 41.8% |
-
-
193469-45-3
5,6-dihydrospiro[imidazo[2,1-b][3]benzazepine-11[11H],4'-piperidine]

-
-
50-00-0
formaldehyd

-
-
100-88-9
cyclohexylsulfamic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydrospiro[imidazo[2,1-b][3]benzazepine-11[11H],4'-piperidine]; formaldehyd With thiophene; hydrogen; palladium 10% on activated carbon In methanol at 50℃; Stage #2: cyclohexylsulfamic acid In acetone | 40% |

-
-
292638-84-7
styrene


-
-
100-88-9
cyclohexylsulfamic acid

-
-
3375-31-3
palladium diacetate

-
-
99254-62-3
N-[2-(3,4-dimethoxyphenyl)ethyl]-2-iodo-5-methoxy-N-methylbenzenepropanamine

| Conditions | Yield |
|---|---|
| In nitrogen; triethylamine | 38% |

-
-
193469-45-3
5,6-dihydrospiro[imidazo[2,1-b][3]benzazepine-11[11H],4'-piperidine]

-
-
100-88-9
cyclohexylsulfamic acid

-
-
1716-42-3
1-(3-chloropropoxy)-4-fluorobenzene

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydrospiro[imidazo[2,1-b][3]benzazepine-11[11H],4'-piperidine]; 1-(3-chloropropoxy)-4-fluorobenzene With sodium carbonate; potassium iodide In 4-methyl-2-pentanone for 18h; Heating / reflux; Stage #2: cyclohexylsulfamic acid In acetone | 37% |

-
-
193469-45-3
5,6-dihydrospiro[imidazo[2,1-b][3]benzazepine-11[11H],4'-piperidine]

-
-
72641-13-5
tetrahydro-2-furanmethanol methanesulfonate (ester)

-
-
100-88-9
cyclohexylsulfamic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydrospiro[imidazo[2,1-b][3]benzazepine-11[11H],4'-piperidine]; tetrahydro-2-furanmethanol methanesulfonate (ester) With sodium carbonate In 4-methyl-2-pentanone Heating / reflux; Stage #2: cyclohexylsulfamic acid In acetone | 20.7% |

-
-
193469-45-3
5,6-dihydrospiro[imidazo[2,1-b][3]benzazepine-11[11H],4'-piperidine]

-
-
98-03-3
thiophene-2-carbaldehyde

-
-
100-88-9
cyclohexylsulfamic acid

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydrospiro[imidazo[2,1-b][3]benzazepine-11[11H],4'-piperidine]; thiophene-2-carbaldehyde With hydrogen; nickel In methanol Stage #2: cyclohexylsulfamic acid In acetone | 6.6% |


| Conditions | Yield |
|---|---|
| (i) MeCN, (ii) /BRN= 2208885/, iPrOH; Multistep reaction; |


| Conditions | Yield |
|---|---|
| (i) THF, (ii) /BRN= 2208885/, acetone; Multistep reaction; |


| Conditions | Yield |
|---|---|
| (i) iPrOH, (ii) /BRN= 2208885/, acetone; Multistep reaction; |

Cyclamic acid Chemical Properties
Chemistry informtions about Cyclamic acid (100-88-9) is:
IUPAC Name: (2E)-2-hydroxyimino-1-phenylpropan-1-one ; -hydroxyimino-1-phenylpropan-1-one
Synonyms: Cyclohexanesulphamic acid ; cyclohexanesulphamicacid ; Cyclohexylamidosulfuric acid ; cyclohexylamidosulfuricacid ; Cyclohexylamidosulphuric acid ; cyclohexylamidosulphuricacid ; Cyclohexylaminesulfonic acid ; cyclohexylaminesulfonicacid
MF: C6H13NO3S
MW: 179.24
EINECS: 202-898-1
Melting Point: ~180 °C (dec.)
Density: 1.32 g/cm3
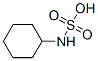
Following is the molecular structure of Cyclamic acid (100-88-9) is:
Cyclamic acid Uses
The sodium and calcium salts of Cyclamic acid (100-88-9) are used as artificial sweeteners under the name cyclamate.This Chemical is banned in the USA and other countries for use as a sweetener. In the UK it used to be banned, but now it is allowed.This is also sometimes considered to be a carcinogen.
Cyclamic acid Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
| mouse | LD50 | intravenous | 180mg/kg (180mg/kg) | | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#01774, |
| mouse | LD50 | oral | 680mg/kg (680mg/kg) | Behavioral: convulsions or effect on seizure threshold lungs, thorax, or respiration: respiratory stimulation gastrointestinal: changes in structure or function of salivary glands | National Technical Information Service. Vol. OTS0540890, |
| rat | LD50 | intravenous | 4gm/kg (4000mg/kg) | | American Journal of the Medical Sciences. Vol. 225, Pg. 551, 1953. |
| rat | LD50 | oral | 12gm/kg (12000mg/kg) | | American Journal of the Medical Sciences. Vol. 225, Pg. 551, 1953. |
Cyclamic acid Safety Profile
Safety Statements:
S22: Do not breathe dust.
S24/25: Avoid contact with skin and eyes.
WGK Germany: 2
RTECS: GV6950000
Related Products
- Cyclamic acid
- 1008-89-5
- 10089-10-8
- 1008-91-9
- 1008-92-0
- 1008-95-3
- 100896-07-9
- 100898-62-2
- 100900-06-9
- 100900-07-0
- 100900-08-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View