-
Name
Cyclobutanecarboxylic acid
- EINECS 223-072-7
- CAS No. 3721-95-7
- Article Data40
- CAS DataBase
- Density 1.202 g/cm3
- Solubility Slightly soluble in water.
- Melting Point -7.5 ºC(lit.)
- Formula C5H8O2
- Boiling Point 195.3 ºC at 760 mmHg
- Molecular Weight 100.117
- Flash Point 85.4 ºC
- Transport Information UN 3265
- Appearance Clear colourless to slightly amber liquid
- Safety 23-24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Cyclobutane-1-carboxylicacid;NSC 4535;Cyclobutance carboxylic acid;
- PSA 37.30000
- LogP 0.87110
Synthetic route

| Conditions | Yield |
|---|---|
| In dichloromethane at -30 - -20℃; for 3h; Temperature; UV-irradiation; | 97% |
| In cyclohexane at 10 - 16℃; Solvent; Diels-Alder Cycloaddition; | 95% |
-
-
1427519-99-0
(3S,4S)-4-(N-allyl-4-methylphenylsulfonamido)-6-methyl-1,3-diphenylhept-1-yn-3-yl cyclobutanecarboxylate

-
A
-
3721-95-7
Cyclobutanecarboxylic acid

-
B
-
1427520-27-1
((3R,6S)-6-isobutyl-3-methyl-5-phenyl-1-tosyl-1,2,3,6-tetrahydropyridin-4-yl)(phenyl)methanone

| Conditions | Yield |
|---|---|
| With Echavarren's catalyst; water In 1,2-dichloro-ethane at 80℃; for 24h; Inert atmosphere; stereoselective reaction; | A n/a B 96% |

| Conditions | Yield |
|---|---|
| With C15H27Br2CoN3; potassium hydroxide In toluene at 140℃; for 16h; Cannizzaro Reaction; Inert atmosphere; Sealed tube; | 73% |
| With lead(IV) acetate; calcium carbonate In benzene |
-
-
124-38-9
carbon dioxide

-
-
4399-47-7
Bromocyclobutane

-
-
584-08-7
potassium carbonate

-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With nickel(II) bromide dimethoxyethane; 2.9-dimethyl-1,10-phenanthroline; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; C60H36N2 In N,N-dimethyl-formamide at 20℃; for 24h; Molecular sieve; Irradiation; | 41% |


| Conditions | Yield |
|---|---|
| With poly(bisanthracenyl) diselenide; dihydrogen peroxide In tert-butyl alcohol for 5h; Heating; | 15% |
| With selenium(IV) oxide; dihydrogen peroxide; tert-butyl alcohol | |
| With dihydrogen peroxide In tert-butyl alcohol |

| Conditions | Yield |
|---|---|
| With silver(l) oxide | |
| With sodium hydroxide at 80℃; for 42h; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide | |
| durch Verseifung; | |
| beim Verseifen; |

| Conditions | Yield |
|---|---|
| at 210 - 220℃; |
-
-
98431-45-9
ethyl 3-iodocyclobutane-1-carboxylate

-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium amalgam; ethanol |
-
-
124-38-9
carbon dioxide

-
-
53213-06-2, 99503-19-2
cyclobutyltriphenylphosphonium ylide

-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| (i) , (ii) (alkaline hydrolysis); Multistep reaction; |
-
-
17684-73-0
2-phenylbicyclo<1.1.1>pentan-2-ol

-
A
-
3721-95-7
Cyclobutanecarboxylic acid

-
B
-
5407-98-7
1-cyclobutyl-1-phenyl-methanone

-
C
-
93061-30-4
bicyclo<1.1.1>pentanone

-
D
-
93039-41-9
3-oxocyclobutyl phenyl ketone

-
E
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With ruthenium tetroxide In tetrachloromethane; acetonitrile for 72h; CCl4/CH3CN/H2O; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) EtOH, H2O; 2.) reflux; Multistep reaction; | |
| Multi-step reaction with 2 steps 1: H3O+ 2: Heating View Scheme |
-
-
79341-47-2
-1,1-Cyclobutandicarbonsaeure-diethyleater

-
A
-
3721-95-7
Cyclobutanecarboxylic acid

-
C
-
42593-04-4
Cyclobutancarbonsaeure

-
D
-
79341-46-1
-1-Cyclobutancarbonsaeure

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1) EtOH, reflux, 2) 160 - 170 deg C; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutyl-ammonium chloride 1) rt, 1 h, 2) distillation; Yield given. Multistep reaction; |
-
-
109-64-8
1,3-dibromo-propane

-
-
105-53-3
diethyl malonate

-
A
-
3721-95-7
Cyclobutanecarboxylic acid

-
B
-
5445-51-2
cyclobutane-1,1'-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride for 1h; Ambient temperature; other subst. 1,3-dibromo compounds, other phase transfer catalysts; | |
| With sodium hydroxide; tetrabutyl-ammonium chloride for 1h; Ambient temperature; Yield given. Yields of byproduct given; |

-
-
14924-53-9
ethyl cyclobutylcarboxylate

-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In dichloromethane for 5h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
7446-08-4
selenium(IV) oxide

-
-
7722-84-1
dihydrogen peroxide

-
-
120-92-3
cyclopentanone

-
-
75-65-0
tert-butyl alcohol

-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
5445-51-2
cyclobutane-1,1'-dicarboxylic acid

-
A
-
3721-95-7
Cyclobutanecarboxylic acid

-
B
-
124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| at 210 - 220℃; |
-
-
30466-31-0
cyclobutanecarboxylic acid phenyl ester

-
A
-
3721-95-7
Cyclobutanecarboxylic acid

-
B
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| at 300℃; |

-
-
7664-93-9
sulfuric acid

-
-
403595-50-6
2-cyano-3-cyclobutyl-3-oxo-propionic acid ethyl ester

-
A
-
141-82-2
malonic acid

-
B
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
120-92-3
cyclopentanone

-
A
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
B
-
13392-69-3
valeric acid

-
C
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dibutyl ether; bis[3,5-bis(trifluoromethyl)diphenyl] diselenide In 2,2,2-trifluoroethanol at 20℃; for 8h; Product distribution; Further Variations:; Catalysts; Solvents; Baeyer-Villiger oxidation; |

-
-
59078-45-4
cyclobutylidenemethanone

-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium perchlorate; water at 25℃; Kinetics; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 13 percent / Pb(OAc)4, 70percent HClO4 / 28 h / Ambient temperature 2: 2N NaOH View Scheme |
-
-
28246-87-9
ethyl 1-cyano-1-cyclobutanecarboxylate

-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Hydrolysis View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: P2O5 / durch Destillation 2: beim Verseifen View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hypochlorous acid 3: silver oxide View Scheme |
-
-
98070-78-1
1-chloromethyl-cyclobutanol

-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: silver oxide View Scheme |

| Conditions | Yield |
|---|---|
| With thionyl chloride In toluene Heating; | 100% |
| With phosphorus trichloride | |
| With thionyl chloride |
-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
41248-13-9
1-hydroxycyclobutane-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: Cyclobutanecarboxylic acid With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at 0 - 20℃; for 18.5h; Metallation; Stage #2: With oxygen In tetrahydrofuran; hexane at 20℃; for 18h; Oxidation; | 100% |
| Stage #1: Cyclobutanecarboxylic acid With n-butyllithium; diisopropylamine In tetrahydrofuran; n-heptane at -20 - 20℃; Stage #2: With oxygen In tetrahydrofuran; n-heptane for 19h; Stage #3: With hydrogenchloride In water |
-
-
542-69-8
1-iodo-butane

-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
58148-13-3
1-butylcyclobutane carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: Cyclobutanecarboxylic acid With lithium diethylamide In tetrahydrofuran; n-heptane; ethylbenzene at 0 - 20℃; for 2h; Stage #2: 1-iodo-butane In tetrahydrofuran; n-heptane; ethylbenzene at 20℃; | 100% |
| With lithium diisopropyl amide In tetrahydrofuran at 20℃; for 12h; | |
| Stage #1: Cyclobutanecarboxylic acid With lithium diisopropyl amide In tetrahydrofuran; hexane at -78 - 20℃; for 1h; Stage #2: 1-iodo-butane In tetrahydrofuran at 0 - 20℃; for 12h; |
-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
144222-22-0
tert-butyl 4-(aminomethyl)piperidine-1-carboxylate

-
-
666853-33-4
C16H28N2O3

| Conditions | Yield |
|---|---|
| With hydrogenchloride; dmap; diethylamine In dichloromethane | 100% |

| Conditions | Yield |
|---|---|
| With tetramethylorthosilicate In toluene at 110℃; for 2h; Inert atmosphere; | 100% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With triethylamine; HATU In dichloromethane at 22℃; for 2h; Inert atmosphere; | 99% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
53-43-0
dehydroepiandrosterone

-
-
1345406-89-4
3β-cyclobutylcarbonyloxy-5-androsten-17-one

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In chloroform at 20℃; for 2h; | 98% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 2h; | 90.4% |
| With dmap; dicyclohexyl-carbodiimide In chloroform at 20℃; for 2h; | 84% |
| With dmap; dicyclohexyl-carbodiimide In chloroform Steglich Esterification; | |
| With dmap; dicyclohexyl-carbodiimide |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 16h; Inert atmosphere; | 98% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 25℃; | 90% |
| With dmap; diisopropyl-carbodiimide In dichloromethane at 20℃; for 5h; | 89% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
616-38-6
carbonic acid dimethyl ester

-
-
765-85-5
methyl cyclobutanecarboxylate

| Conditions | Yield |
|---|---|
| With diiron nonacarbonyl at 180℃; for 1h; Sealed tube; | 98% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
743478-65-1
cyclobutylmalonyl Dichloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In triethylamine at 55℃; for 120h; Heating / reflux; Nitrogen atmosphere; | 97% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In toluene for 1h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether for 1.5h; Ambient temperature; | 96% |
| With lithium aluminium tetrahydride In diethyl ether | 72% |
| With lithium aluminium tetrahydride; diethyl ether |
-
-
3721-95-7
Cyclobutanecarboxylic acid


| Conditions | Yield |
|---|---|
| Stage #1: Cyclobutanecarboxylic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 0.5h; Stage #2: (2S,3R,4S,5S)-ethyl 5-(2-(azetidin-1-yl)pyridin-3-yl)-3-(tert-butyl)-4-nitropyrrolidine-2-carboxylate With triethylamine In dichloromethane for 0.5h; Cooling with ice; | 96% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
797-63-7
levonorgestrel

-
-
86679-36-9
D-(-)-norgestrel 17β-cyclobutanecarboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate; trifluoroacetic anhydride In benzene for 1.5h; Ambient temperature; | 95.4% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
74-88-4
methyl iodide

-
-
32936-76-8
1-methylcyclobutane carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: Cyclobutanecarboxylic acid With lithium diisopropyl amide In tetrahydrofuran; hexane at 5℃; for 0.25h; Stage #2: methyl iodide In tetrahydrofuran; hexane at 20℃; | 95% |
| Stage #1: Cyclobutanecarboxylic acid With lithium diisopropyl amide In tetrahydrofuran at 0℃; for 1h; Stage #2: methyl iodide In tetrahydrofuran at 20℃; | 92.1% |
| Stage #1: Cyclobutanecarboxylic acid With lithium diisopropyl amide In tetrahydrofuran at 0℃; for 0.5h; Stage #2: methyl iodide In tetrahydrofuran at 20℃; | 92.1% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In toluene for 1h; Inert atmosphere; | 95% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 94.6% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
765-85-5
methyl cyclobutanecarboxylate

| Conditions | Yield |
|---|---|
| In diethyl ether for 0.5h; Ambient temperature; | 94% |
-
-
3721-95-7
Cyclobutanecarboxylic acid


| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 0.5h; | 94% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In toluene for 1h; Inert atmosphere; | 94% |
-
-
933043-85-7
2-[2-(3-chloro-phenyl)-2H-tetrazol-5-yl]-piperidine hydrochloride

-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In dichloromethane at 25℃; for 20h; | 93% |

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 25℃; for 1h; | 93% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
1416881-52-1
(4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile

| Conditions | Yield |
|---|---|
| With caesium carbonate In dimethyl sulfoxide Inert atmosphere; Irradiation; | 93% |
-
-
141-30-0
3,6-dichlorpyridazine

-
-
3721-95-7
Cyclobutanecarboxylic acid

-
-
107228-57-9
3,6-dichloro-4-cyclobutylpyridazine

| Conditions | Yield |
|---|---|
| Stage #1: 3,6-dichlorpyridazine; Cyclobutanecarboxylic acid With sulfuric acid; silver nitrate In water at 70℃; for 0.0166667h; Stage #2: With ammonium peroxydisulfate In water at 20℃; for 0.416667 - 0.583333h; Stage #3: With ammonia In water at 5℃; | 92% |
| With ammonium persulfate; sulfuric acid; silver nitrate In water | |
| With ammonium persulfate; sulfuric acid; silver nitrate In water | |
| With ammonium peroxydisulfate; sulfuric acid; silver nitrate In water at 72℃; |
-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In toluene for 1h; Inert atmosphere; | 92% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In toluene for 1h; Inert atmosphere; | 92% |
-
-
3721-95-7
Cyclobutanecarboxylic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In toluene for 1h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With 6,6'-dimethyl-2,2'-bipyridine; nickel(II) bromide trihydrate; di-tert-butyl dicarbonate; sodium iodide; magnesium chloride; zinc In N,N-dimethyl acetamide at 30℃; for 24h; regioselective reaction; | 91% |
Cyclobutanecarboxylic acid Specification
The Cyclobutanecarboxylic acid, with the CAS registry number 3721-95-7, is also known as Cyclo-butyl formic acid. It belongs to the product categories of Acids and Derivatives; Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Cyclobutanes & Cyclobutenes; Simple 4-Membered Ring Compounds; Cycloalkanes; C1 to C5; Carbonyl Compounds; Carboxylic Acids. Its EINECS registry number is 223-072-7. This chemical's molecular formula is C5H8O2 and molecular weight is 100.12. What's more, its IUPAC name is the same with its product name. This chemical can be prepared by Cyclobutane-1, 1-dicarboxylic acid. This reaction needs the temperature of 160 °C. The yield is 86-91 %.
Physical properties about Cyclobutanecarboxylic acid are: (1)ACD/LogP: 0.65; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.14; (4)ACD/LogD (pH 7.4): -1.93; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 8.84; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.501; (14)Molar Refractivity: 24.55 cm3; (15)Molar Volume: 83.2 cm3; (16)Surface Tension: 49.5 dyne/cm; (17)Density: 1.202 g/cm3; (18)Flash Point: 85.4 °C; (19)Enthalpy of Vaporization: 47.56 kJ/mol; (20)Boiling Point: 195.3 °C at 760 mmHg; (21)Vapour Pressure: 0.182 mmHg at 25 °C.
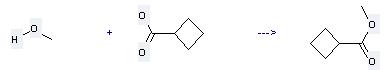
Uses of Cyclobutanecarboxylic acid: (1) it is used in organic synthesis; (2) it is used to produce other chemicals. For example, it can react with Methanol to get Cyclobutanecarboxylic acid methyl ester. The reaction occurs with reagent H2SO4 and other condition of heating for 8 hours. The yield is 75 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. Therefore, you should avoid contacting with skin, eyes and wear suitable protective clothing. You can not breathe the gas/fumes/vapour/spray. And in case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(O)C1CCC1
(2) InChI: InChI=1S/C5H8O2/c6-5(7)4-2-1-3-4/h4H,1-3H2,(H,6,7)
(3) InChIKey: TXWOGHSRPAYOML-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1270mg/kg (1270mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 21, Pg. 85, 1969. Link to PubMed | |
| mouse | LD50 | subcutaneous | 1270mg/kg (1270mg/kg) | Journal of Pharmacy and Pharmacology. Vol. 21, Pg. 85, 1969. Link to PubMed |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View