-
Name
Dehydrocholic acid
- EINECS 201-335-7
- CAS No. 81-23-2
- Article Data35
- CAS DataBase
- Density 1.172 g/cm3
- Solubility 10 mg/mL in ethanol
- Melting Point 238-240 °C
- Formula C24H34O5
- Boiling Point 581.5 °C at 760 mmHg
- Molecular Weight 402.531
- Flash Point 319.5 °C
- Transport Information
- Appearance white to off-white amorphous powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 5b-Cholan-24-oic acid,3,7,12-trioxo- (8CI);3,7,12-Tri-keto-5b-Cholan-24-oic acid;3,7,12-Triketo-5b-cholanic acid;3,7,12-Triketocholanic acid;3,7,12-Trioxo-5b-cholan-24-oic acid;3,7,12-Trioxo-5b-cholanic acid;3,7,12-Trioxocholanic acid;Acolen;Bilidren;Bilostat;Cholagon;Cholan DH;Cholepatin;Cholic acid, dehydro-;Cholimed;Chologon;DHC;Decholin;Dehychol;Dehycon;
- PSA 88.51000
- LogP 4.07330
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium perchlorate; sodium chloride In water at 25℃; for 40h; pH=12; Electrochemical reaction; 50 mA; | 100% |
| With sodium bromate; ammonium cerium (IV) nitrate In water; acetonitrile at 80℃; for 3h; Temperature; Green chemistry; | 95% |
| With sodium bromate; ammonium cerium (IV) nitrate In water; acetonitrile at 80℃; for 3.3h; | 95% |

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With jones' reagent In acetone for 0.0833333h; | 100% |

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Stage #1: C27H46O5 With potassium sulfate; cerous nitrate at 8℃; for 2.33333h; Large scale; Stage #2: With Tetradecanoic acid 1-methylethyl ester for 3h; Temperature; Large scale; | 94% |
-
-
81-25-4
cholic acid

-
A
-
2458-08-4
3α,7α-dihydroxy-12-oxo-5β-cholan-24-oic acid

-
B
-
911-40-0
7-ketodeoxycholic acid

-
C
-
517-33-9
7,12-dioxolitocholic acid

-
D
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium perchlorate; sodium chloride In water at 25℃; for 6h; pH=12; Product distribution; Further Variations:; electricity; Electrochemical reaction; 200 mA; | A 10% B 33% C 46% D 11% |

-
-
1100696-11-4
(24RS,25RS)-3α,7α,12α,24,26-pentahydroxy-5β-cholestan-27-yl sodium sulfate

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide In water; acetic acid at 25℃; for 5h; | 90 mg |

| Conditions | Yield |
|---|---|
| With Pseudomonas fluorescens B26 broth In water at 30℃; for 48h; | 41 % Chromat. |
-
-
81-25-4
cholic acid

-
A
-
911-40-0
7-ketodeoxycholic acid

-
B
-
517-33-9
7,12-dioxolitocholic acid

-
C
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| unter aeroben Bedingungen; |

-
-
1061-64-9, 23605-03-0, 73804-37-2, 73837-07-7, 73889-90-4, 85552-41-6, 85552-42-7, 85552-43-8, 85552-44-9
(25Ξ)-3ξ.7ξ.12ξ.24ξ-tetrahydroxy-5β-cholestanoic acid-(26)

-
-
64-19-7
acetic acid

-
-
81-23-2
dehydrocholic acid

-
-
19964-67-1
(R)-methyl-4-((5R,8R,9S,10S,13R,14S,17R)-3,3-dimethoxy-10,13-dimethyl-7,12-dioxohexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate

-
-
81-23-2
dehydrocholic acid

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; water In ethyl acetate pH=1 - 5; |


-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium hydride / tetrahydrofuran / 0.17 h / 15 °C 1.2: 0.5 h / 15 °C 2.1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 3.1: sodium hydroxide / methanol / 2 h / 60 °C 4.1: Jones reagent / acetone / 20 °C View Scheme |

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 2: sodium hydroxide / methanol / 2 h / 60 °C 3: Jones reagent / acetone / 20 °C View Scheme |

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / methanol / 2 h / 60 °C 2: Jones reagent / acetone / 20 °C View Scheme |
-
-
71883-64-2
cholic acid

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With Jones reagent In acetone at 20℃; | 20 mg |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 5 °C 2.1: palladium 10% on activated carbon; hydrogen / pyridine / 12 h / 25 °C / 2585.81 Torr 3.1: lithium tri-t-butoxyaluminum hydride / tetrahydrofuran / 0.5 h / 5 °C 4.1: toluene-4-sulfonic acid / toluene / 2 h / 120 °C / Dean-Stark 5.1: sodium L-ascorbate; oxygen; tetrakis(actonitrile)copper(I) hexafluorophosphate / acetone; methanol / 2.17 h / 25 - 50 °C / 775.74 Torr 6.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / 70 °C / Inert atmosphere 6.2: 2 h / 70 °C / Inert atmosphere 7.1: dmap; triethylamine / dichloromethane / 7 h / 25 °C 8.1: boron trifluoride diethyl etherate / dichloromethane / 2 h / 0 - 15 °C 9.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C 9.2: -78 - 15 °C 10.1: sodium hydride / tetrahydrofuran / 0.17 h / 15 °C 10.2: 0.5 h / 15 °C 11.1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 12.1: sodium hydroxide / methanol / 2 h / 60 °C 13.1: Jones reagent / acetone / 20 °C View Scheme |
-
-
50788-88-0
6α,7α-dihydrooxireno<6,7>androst-4-ene-3,17-dione

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: palladium 10% on activated carbon; hydrogen / pyridine / 12 h / 25 °C / 2585.81 Torr 2.1: lithium tri-t-butoxyaluminum hydride / tetrahydrofuran / 0.5 h / 5 °C 3.1: toluene-4-sulfonic acid / toluene / 2 h / 120 °C / Dean-Stark 4.1: sodium L-ascorbate; oxygen; tetrakis(actonitrile)copper(I) hexafluorophosphate / acetone; methanol / 2.17 h / 25 - 50 °C / 775.74 Torr 5.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / 70 °C / Inert atmosphere 5.2: 2 h / 70 °C / Inert atmosphere 6.1: dmap; triethylamine / dichloromethane / 7 h / 25 °C 7.1: boron trifluoride diethyl etherate / dichloromethane / 2 h / 0 - 15 °C 8.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C 8.2: -78 - 15 °C 9.1: sodium hydride / tetrahydrofuran / 0.17 h / 15 °C 9.2: 0.5 h / 15 °C 10.1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 11.1: sodium hydroxide / methanol / 2 h / 60 °C 12.1: Jones reagent / acetone / 20 °C View Scheme |
-
-
91378-51-7
7α-hydroxy-5β-androstane-3,17-dione

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: lithium tri-t-butoxyaluminum hydride / tetrahydrofuran / 0.5 h / 5 °C 2.1: toluene-4-sulfonic acid / toluene / 2 h / 120 °C / Dean-Stark 3.1: sodium L-ascorbate; oxygen; tetrakis(actonitrile)copper(I) hexafluorophosphate / acetone; methanol / 2.17 h / 25 - 50 °C / 775.74 Torr 4.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / 70 °C / Inert atmosphere 4.2: 2 h / 70 °C / Inert atmosphere 5.1: dmap; triethylamine / dichloromethane / 7 h / 25 °C 6.1: boron trifluoride diethyl etherate / dichloromethane / 2 h / 0 - 15 °C 7.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C 7.2: -78 - 15 °C 8.1: sodium hydride / tetrahydrofuran / 0.17 h / 15 °C 8.2: 0.5 h / 15 °C 9.1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 10.1: sodium hydroxide / methanol / 2 h / 60 °C 11.1: Jones reagent / acetone / 20 °C View Scheme |
-
-
64144-66-7
(3α,5β,7α)-3,7-dihydroxyandrostan-17-one

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: toluene-4-sulfonic acid / toluene / 2 h / 120 °C / Dean-Stark 2.1: sodium L-ascorbate; oxygen; tetrakis(actonitrile)copper(I) hexafluorophosphate / acetone; methanol / 2.17 h / 25 - 50 °C / 775.74 Torr 3.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / 70 °C / Inert atmosphere 3.2: 2 h / 70 °C / Inert atmosphere 4.1: dmap; triethylamine / dichloromethane / 7 h / 25 °C 5.1: boron trifluoride diethyl etherate / dichloromethane / 2 h / 0 - 15 °C 6.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C 6.2: -78 - 15 °C 7.1: sodium hydride / tetrahydrofuran / 0.17 h / 15 °C 7.2: 0.5 h / 15 °C 8.1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 9.1: sodium hydroxide / methanol / 2 h / 60 °C 10.1: Jones reagent / acetone / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1.1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 2,3-dicyano-5,6-dichloro-p-benzoquinone / dichloromethane / 48 h / 0 - 25 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 24 h / 5 °C 3.1: palladium 10% on activated carbon; hydrogen / pyridine / 12 h / 25 °C / 2585.81 Torr 4.1: lithium tri-t-butoxyaluminum hydride / tetrahydrofuran / 0.5 h / 5 °C 5.1: toluene-4-sulfonic acid / toluene / 2 h / 120 °C / Dean-Stark 6.1: sodium L-ascorbate; oxygen; tetrakis(actonitrile)copper(I) hexafluorophosphate / acetone; methanol / 2.17 h / 25 - 50 °C / 775.74 Torr 7.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / 70 °C / Inert atmosphere 7.2: 2 h / 70 °C / Inert atmosphere 8.1: dmap; triethylamine / dichloromethane / 7 h / 25 °C 9.1: boron trifluoride diethyl etherate / dichloromethane / 2 h / 0 - 15 °C 10.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C 10.2: -78 - 15 °C 11.1: sodium hydride / tetrahydrofuran / 0.17 h / 15 °C 11.2: 0.5 h / 15 °C 12.1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 13.1: sodium hydroxide / methanol / 2 h / 60 °C 14.1: Jones reagent / acetone / 20 °C View Scheme |

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: sodium L-ascorbate; oxygen; tetrakis(actonitrile)copper(I) hexafluorophosphate / acetone; methanol / 2.17 h / 25 - 50 °C / 775.74 Torr 2.1: potassium tert-butylate / tetrahydrofuran / 0.5 h / 70 °C / Inert atmosphere 2.2: 2 h / 70 °C / Inert atmosphere 3.1: dmap; triethylamine / dichloromethane / 7 h / 25 °C 4.1: boron trifluoride diethyl etherate / dichloromethane / 2 h / 0 - 15 °C 5.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C 5.2: -78 - 15 °C 6.1: sodium hydride / tetrahydrofuran / 0.17 h / 15 °C 6.2: 0.5 h / 15 °C 7.1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 8.1: sodium hydroxide / methanol / 2 h / 60 °C 9.1: Jones reagent / acetone / 20 °C View Scheme |

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: dmap; triethylamine / dichloromethane / 7 h / 25 °C 2.1: boron trifluoride diethyl etherate / dichloromethane / 2 h / 0 - 15 °C 3.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 0.5 h / -78 °C 3.2: -78 - 15 °C 4.1: sodium hydride / tetrahydrofuran / 0.17 h / 15 °C 4.2: 0.5 h / 15 °C 5.1: hydrogen; palladium on activated charcoal / ethanol / 4 h / 25 °C / 2585.81 Torr 6.1: sodium hydroxide / methanol / 2 h / 60 °C 7.1: Jones reagent / acetone / 20 °C View Scheme |
-
-
81-23-2
dehydrocholic acid

-
-
108-98-5
thiophenol

-
-
69577-96-4
(R)-4-((5S,8R,9S,10S,13R,14S,17R)-10,13-Dimethyl-3,7,12-trioxo-hexadecahydro-cyclopenta[a]phenanthren-17-yl)-pentanethioic acid S-phenyl ester

| Conditions | Yield |
|---|---|
| With pyridine; O-phenyl phosphorodichloridate In 1,2-dimethoxyethane for 16h; Ambient temperature; | 95% |
| (i) Me2NPOCl2, Et3N, (ii) /BRN= 506523/; Multistep reaction; |
-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With iron(III) chloride; sodium hydroxide In water at 35℃; pH=8; | 95% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; | 94% |

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 70℃; for 5h; | 93% |
| With N-Bromosuccinimide at 70℃; for 1h; Time; | 89% |
| With sulfuric acid | |
| toluene-4-sulfonic acid In benzene Heating; |

| Conditions | Yield |
|---|---|
| With potassium phosphate; D-glucose; diothiothreitol In ethanol for 9h; Ambient temperature; NADH, 7α-hydroxysteroid dehydrogenase, glucose dehydrogenase; pH 6.8; | 91% |
| Multi-step reaction with 2 steps 1: 30 h / Heating 2: NaBH4, 5percent aq. NaOH / 1 h / Ambient temperature View Scheme |
-
-
14174-09-5
dibenzo-24-crown ether

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With tributylphosphine; dicyclohexyl-carbodiimide In chloroform at 0℃; for 3h; | 91% |


| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 3h; | 91% |
-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With dmap; copper diacetate In acetonitrile at 80℃; for 3h; Schlenk technique; Sealed tube; | 91% |
-
-
81-23-2
dehydrocholic acid

-
-
81873-91-8
3α,7β-dihydroxy-12-oxo-5β-cholane-24-oic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; NAD; sodium formate; NADPH at 24℃; for 12h; pH=6; Enzymatic reaction; | 90.6% |
| With glucose dehydrogenase; D-glucose; NAD-dependent 3α-hydroxysteroid dehydrogenase from Pseudomonas testosteroni; NADP-dependent 7β-hydroxysteroid dehydrogenase from Collinsella aerofaciens; NADPH; NADH; magnesium chloride In aq. buffer at 25℃; for 19h; pH=8; Kinetics; Catalytic behavior; pH-value; Time; Enzymatic reaction; stereoselective reaction; | 80 g |
-
-
67-56-1
methanol

-
-
81-23-2
dehydrocholic acid

-
-
19964-67-1
(R)-methyl-4-((5R,8R,9S,10S,13R,14S,17R)-3,3-dimethoxy-10,13-dimethyl-7,12-dioxohexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 4h; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; formaldehyd; diothiothreitol In ethanol for 12h; Ambient temperature; NADH, 3α-hydroxysteroid dehydrogenase, formate dehydrogenase; pH 6.8; | 89% |
| With acetic acid; platinum Hydrogenation; | |
| With sodium hydroxide; platinum Hydrogenation; |
-
-
14949-00-9
5-amino-1, 3, 4-thiadiazole-2-sulfonamide

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 20℃; for 12h; | 89% |
| With thionyl chloride In tetrahydrofuran for 12h; | 82% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 60 - 70℃; pH=7 - 8; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: Iodoethanol; dehydrocholic acid With diisopropyl-carbodiimide In dichloromethane at 20℃; for 0.25h; Steglich Esterification; Inert atmosphere; Stage #2: With dmap In dichloromethane at 20℃; for 14h; Steglich Esterification; | 88% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; D-glucose; diothiothreitol In ethanol for 26h; Ambient temperature; NADPH, 12α-hydroxysteroid dehydrogenase, glucose dehydrogenase; pH 6.8; | 87% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; formaldehyd; diothiothreitol In ethanol for 25h; Ambient temperature; NADH, 3β-hydroxysteroid dehydrogenase, formate dehydrogenase; pH 6.8; | 86% |
-
-
81-23-2
dehydrocholic acid

-
-
102649-81-0
7β-hydroxy-3,12-dioxo-5β-cholan-24-oic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; D-glucose; diothiothreitol In ethanol for 30h; Ambient temperature; NADPH, 7β-hydroxysteroid dehydrogenase, glucose dehydrogenase; pH 6.8; | 86% |

| Conditions | Yield |
|---|---|
| In water for 5 - 6h; Heating / reflux; | 86% |
-
-
81-23-2
dehydrocholic acid

-
-
149-73-5
trimethyl orthoformate


-
B
-
19964-67-1
(R)-methyl-4-((5R,8R,9S,10S,13R,14S,17R)-3,3-dimethoxy-10,13-dimethyl-7,12-dioxohexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoate

| Conditions | Yield |
|---|---|
| Stage #1: dehydrocholic acid With toluene-4-sulfonic acid In methanol for 4h; Reflux; Stage #2: trimethyl orthoformate at 40℃; for 4.2h; Temperature; | A 84% B 7% |

-
-
81-23-2
dehydrocholic acid

| Conditions | Yield |
|---|---|
| With ammonium acetate; sodium cyanoborohydride In methanol Ambient temperature; | 82% |
-
-
81-23-2
dehydrocholic acid

-
-
98-80-6
phenylboronic acid

-
-
5739-38-8
1-(4-methoxyphenyl)-3-phenylpropan-1-one

| Conditions | Yield |
|---|---|
| With P(p-CH3OC6H4)3; 2,2-dimethylpropanoic anhydride In tetrahydrofuran; water at 60℃; for 16h; | 81% |

| Conditions | Yield |
|---|---|
| With P(p-CH3OC6H4)3; 2,2-dimethylpropanoic anhydride In tetrahydrofuran; water at 60℃; for 16h; | 81% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane | 80% |
-
-
81-23-2
dehydrocholic acid

-
-
30516-87-1
3'-azido-2',3'-deoxythymidine

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran for 72h; | 78% |

| Conditions | Yield |
|---|---|
| Stage #1: (2-furyl)methyl alcohol; dehydrocholic acid With dmap In dichloromethane at 0℃; for 0.5h; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 76.7% |

| Conditions | Yield |
|---|---|
| Stage #1: dehydrocholic acid; benzyl alcohol With dmap In dichloromethane at 0℃; for 0.5h; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 76.5% |

| Conditions | Yield |
|---|---|
| Stage #1: piperonol; dehydrocholic acid With dmap In dichloromethane at 0℃; for 0.5h; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 75.6% |
Dehydrocholic acid Chemical Properties
IUPAC Name: (4R)-4-[(5S,8R,9S,10S,13R,14S,17R)-10,13-Dimethyl-3,7,12-trioxo-1,2,4,5,6,8,9,11,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-17-yl]pentanoic Acid
Formula: C24H34O5
Molecular weight: 402.52 g/mol
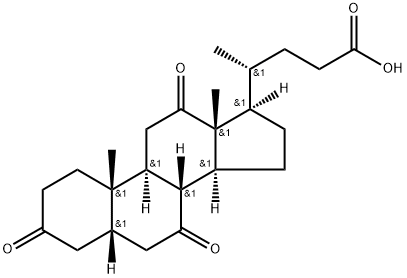
The Molecular Structure of Dehydrocholic acid (CAS NO.81-23-2):
Density: 1.172 g/cm3
Melting Point: 238-240 °C
Flash Point: 319.5 °C
Boiling Point: 581.5 °C at 760 mmHg
Index of Refraction: 1.534
Molar Refractivity: 106.86 cm3
Molar Volume: 343.3 cm3
Surface Tension: 45.4 dyne/cm
Enthalpy of Vaporization: 95 kJ/mol
Vapour Pressure: 4.96E-15 mmHg at 25 °C
Solubility Ethanol: 10 mg/mL
Appearance: white to off-white amorphous powder
XLogP3-AA: 2.6
H-Bond Donor: 1
H-Bond Acceptor: 5
Rotatable Bond Count: 4
Tautomer Count: 18
Exact Mass: 402.240624
MonoIsotopic Mass: 402.240624
Topological Polar Surface Area: 88.5
Heavy Atom Count: 29
Canonical SMILES: CC(CCC(=O)O)C1CCC2C1(C(=O)CC3C2C(=O)CC4C3(CCC(=O)C4)C)C
InChI: InChI=1S/C24H34O5/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26/h13-14,16-18,22H,4-12H2,1-3H3,(H,28,29)/t13-,14+,16-,17+,18+,22+,23+,24-/m1/s1
InChIKey: OHXPGWPVLFPUSM-KLRNGDHRSA-N
EINECS: 201-335-7
Product Categories: Bile Acids;Biochemistry;Steroids
Dehydrocholic acid Uses
Dehydrocholic acid (CAS NO.81-23-2) can be used as a gallbladder drug and very useful for the gallbladder and biliary tract dysfunction, post-cholecystectomy syndrome, chronic cholecystitis and cholelithiasis and chronic hepatitis.
Dehydrocholic acid Production
Dehydrocholic acid is manufactured by the oxidation of cholic acid.
Dehydrocholic acid Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 1492mg/kg (1492mg/kg) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Japanese Journal of Pharmacology. Vol. 22, Pg. 235, 1972. |
| mouse | LD50 | oral | 3100mg/kg (3100mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 12, Pg. 857, 1962. | |
| mouse | LD50 | subcutaneous | 1620mg/kg (1620mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 116, Pg. 154, 1958. | |
| rat | LD50 | intramuscular | 1500mg/kg (1500mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 222, Pg. 244, 1954. | |
| rat | LD50 | intravenous | 750mg/kg (750mg/kg) | Drugs in Japan Vol. 6, Pg. 495, 1982. | |
| rat | LD50 | oral | 4gm/kg (4000mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 116, Pg. 154, 1958. |
Dehydrocholic acid Consensus Reports
Reported in EPA TSCA inventory.
Dehydrocholic acid Safety Profile
Safety Statements: 24/25
S24/25:Avoid contact with skin and eyes.
WGK Germany: 2
RTECS: FZ2300000
Moderately toxic by ingestion, intravenous, intramuscular, and subcutaneous routes. When heated to decomposition it emits acrid smoke and fumes.
Dehydrocholic acid Specification
The chemical synonyms of Dehydrocholic acid (CAS NO.81-23-2) are 3,7,12-Triketo-5beta-cholanoic acid ; 3,7,12-Triketocholanic acid ; 3,7,12-Trioxo-5-beta-cholan-24-oic acid ; 3,7,12-Trioxo-5beta-cholan-24-oic acid ; 3,7,12-Trioxo-5beta-cholanic acid ; 3,7,12-Trioxocholanic acid ; 4-10-00-03478 (Beilstein Handbook Reference) ; 5beta-Cholanic acid, 3,7,12-trioxo- ; Acide dehydrocholique ; Acide dehydrocholique [French] ; Acide dehydrocholique [INN-French] ; Acido dehidrocolico ; Acido dehidrocolico [INN-Spanish] ; Acido dehidrocolico [Spanish] ; Acido deidrocolico ; Acido deidrocolico [DCIT] ; Acidum dehydrocholicum ; Acidum dehydrocholicum [INN-Latin] ; Chologon ; DHC ; Decholin ; Dee-Co ; Dehychol ; Erebile ; Felacrinos ; Hykolex ; Ketochol ; Ketocholanic acid ; Khologon ; NSC 8796 ; Novocolin ; Oxycholin ; Procholon ; Sanocholen ; Triketocholanic acid ; UNII-NH5000009I . Dehydrocholic acid (CAS NO.81-23-2) is dissolved in sodium hydroxide, slightly soluble in chloroform, slightly soluble in ethanol, practically insoluble in water.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View