-
Name
Dibenzylamine
- EINECS 203-117-7
- CAS No. 103-49-1
- Article Data700
- CAS DataBase
- Density 1.026 g/cm3
- Solubility soluble in alcohol and ether, insoluble in water
- Melting Point -26 °C(lit.)
- Formula C14H15N
- Boiling Point 300 °C at 760 mmHg
- Molecular Weight 197.28
- Flash Point 143.3 °C
- Transport Information UN 2810
- Appearance Colorless to light yellow liquid
- Safety 26-61-45-36/37/39
- Risk Codes 22-36/38-52/53-34
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi,
Xi, C
C
- Synonyms Dibenzylamine(8CI);(N-Benzylaminomethyl)benzene;Bibenzylamine;DBA;N,N-Dibenzylamine;N-(Phenylmethyl)benzenemethanamine;N-Benzylbenzylamine;NSC 4811;
- PSA 12.03000
- LogP 3.36730
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; Ru((R,R)-cyP2N2)HCl In benzene-d6 at 20℃; under 2280.15 Torr; for 4h; Product distribution / selectivity; Alkaline conditions; Cooling with liquid nitrogen; | 100% |
| With hydrogen; Ru((R,R)-cyP2(NH)2)HCl In benzene-d6 at 20℃; under 2280.15 Torr; for 4h; Product distribution / selectivity; Alkaline conditions; Cooling with liquid nitrogen; | 100% |
| With bis(pentamethylcyclopentadienyl)zinc; hydrogen; 1,3-bis(2,4,6-trimethylphenyl)imidazolidin-2-ylidene In benzene-d6 at 25℃; under 75007.5 Torr; for 72h; Reagent/catalyst; Temperature; Time; Pressure; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With indium; ammonium chloride In ethanol for 3.5h; Product distribution; Further Variations:; Reagents; Heating; | 100% |
| With indium(III) chloride; ammonium chloride; zinc In ethanol; water for 3.5h; Catalytic behavior; Reagent/catalyst; Reflux; chemoselective reaction; | 100% |
| With titanium(III) chloride In methanol Ambient temperature; | 93% |
| With hydrogen iodide | |
| With phosphorus trichloride durch Zersetzen des Reaktionsproduktes mit Wasser; |
-
-
27845-50-7
(E)-N-benzylidenebenzylamine

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With calcium hydride; zinc dibromide In tetrahydrofuran at 40℃; for 12h; | 100% |
| With hydrogen; β-cyclodextrin/Pd In water at 25℃; under 15001.2 Torr; for 1h; | 100% |
| With hydrogen; palladium on activated charcoal In tetrahydrofuran at 25℃; under 15001.2 Torr; | 92% |

| Conditions | Yield |
|---|---|
| With 1,1,3,3-Tetramethyldisiloxane; [((CH3)5C5)IrCl((CH3)2NC6H3C5H4N)]; trityl tetrakis(pentafluorophenyl)borate In 1,1,2,2-tetrachloroethane at 100℃; for 0.5h; Inert atmosphere; Schlenk technique; Glovebox; chemoselective reaction; | 100% |
| With indium(III) bromide; 1,1,3,3-Tetramethyldisiloxane In 5,5-dimethyl-1,3-cyclohexadiene at 140℃; for 3h; Solvent; Temperature; Sealed tube; | 91% |
| With 1,1,3,3-Tetramethyldisiloxane; C25H23N3O2; copper(II) bis(trifluoromethanesulfonate) In toluene at 65℃; for 24h; Inert atmosphere; | 90% |
-
-
27046-29-3
N-(4-bromobenzylidene)benzylamine

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 30℃; under 22502.3 Torr; | 100% |

| Conditions | Yield |
|---|---|
| With Au0998Ag0002; hydrogen In ethanol at 90℃; under 6080.41 Torr; for 24h; chemoselective reaction; | 99% |
| With butyl triphenylphosphonium tetraborate at 20℃; for 0.166667h; | 98% |
| With benzyltriphenylphosphonium borohydride In methanol at 20℃; for 0.333333h; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 90℃; under 750.075 Torr; for 20h; chemoselective reaction; | 99% |
| With hydrogen In water at 120℃; under 37503.8 Torr; for 24h; chemoselective reaction; | 98% |
| With sodium tetrahydroborate; nickel dichloride In methanol at 20℃; for 4h; Reduction; | 96% |

| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 60℃; under 7500.75 Torr; for 8h; Autoclave; | 99% |
| With hydrogen at 140℃; under 2250.23 Torr; for 24h; Reagent/catalyst; Pressure; Molecular sieve; | 98% |
| With 5 % Pd/TiO2 at 30℃; for 10h; Catalytic behavior; Inert atmosphere; UV-irradiation; | 96% |
-
-
905244-84-0
N,N-dibenzyl-2,4,6-triisopropylbenzenesulfonamide

-
A
-
717-74-8
1,3,5-triisopropyl benzene

-
B
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate; chloro-trimethyl-silane; magnesium In tetrahydrofuran at 50℃; Inert atmosphere; | A 99% B 93% |

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With potassium carbonate; mercaptoacetic acid In methanol at 0 - 25℃; Inert atmosphere; chemoselective reaction; | 99% |
-
-
1587717-84-7
(R)-N,N-dibenzyl-2-methylpropane-2-sulfinamide

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With water; iodine In tetrahydrofuran at 50℃; | 99% |

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate; chloro-trimethyl-silane; magnesium In tetrahydrofuran at 50℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With 1,2-bis-(diphenylphosphino)ethane; palladium dichloride; lithium hydroxide In neat (no solvent) at 100℃; for 24h; | 98% |
| With C42H44ClN4P2Ru(1+)*Cl(1-); potassium tert-butylate In tert-Amyl alcohol at 120℃; for 24h; | 98% |
| In neat (no solvent) at 145℃; for 24h; Inert atmosphere; Sealed tube; | 97% |

| Conditions | Yield |
|---|---|
| With benzaldehyde; triethylamine | 98% |

| Conditions | Yield |
|---|---|
| With 40% potassium fluoride/alumina for 0.0666667h; Microwave irradiation; Neat (no solvent); | A 98% B 95% |
-
-
140-11-4
Benzyl acetate

-
-
100-46-9
benzylamine

-
A
-
588-46-5
N-(phenylmethyl)acetamide

-
B
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; sodium acetate In neat (no solvent) at 115℃; for 24h; Inert atmosphere; Glovebox; Green chemistry; | A 98% B 67% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; for 2h; | 97% |
| With ethanol; nickel at 100℃; under 73550.8 Torr; Hydrogenation; | |
| With sodium acetate durch elektrolytische Reduktion an einer Blei-Kathode; |
-
-
10479-30-8
N,N-dibenzylacetamide

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With ammonium bromide; ethylenediamine at 80℃; for 10h; Reagent/catalyst; Solvent; Microwave irradiation; | 97% |
| Stage #1: N,N-dibenzylacetamide With Schwartz's reagent In tetrahydrofuran at 20℃; for 0.05h; Inert atmosphere; Stage #2: With water In tetrahydrofuran Inert atmosphere; | 94% |
-
-
1285711-92-3
N,N-dibenzyl-3-methyl-2-pyridinamine

-
A
-
6456-92-4
1,3-dimethylpyridin-2(1H)-one

-
B
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dibenzyl-3-methyl-2-pyridinamine With methyl trifluoromethanesulfonate In dichloromethane at 0 - 4℃; Stage #2: With sodium hydroxide In methanol at 50℃; for 6h; | A 86% B 97% |
-
-
14618-33-8
2,2,2-trifluoro-N,N-bis(phenylmethyl)acetamide

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 96% |
| With ammonium iodide; hydrazine In ethanol at 50℃; for 36h; | 96% |
| With ammonium bromide; ethylenediamine at 50℃; for 3h; Microwave irradiation; Inert atmosphere; neat (no solvent); | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: benzaldehyde With lithium perchlorate; 1,1,1,3,3,3-hexamethyl-disilazane at 50℃; for 0.5h; Stage #2: With sodium tetrahydroborate In methanol at 20℃; for 2h; | 95% |
| Stage #1: benzaldehyde With titanium(IV) isopropylate; ammonium chloride; triethylamine In ethanol at 20℃; for 6h; Stage #2: With sodium tetrahydroborate In ethanol at 20℃; for 3h; Further stages.; | 76% |
| With ammonium acetate; borohydride exchange resin; triethylamine hydrochloride In ethanol for 0.5h; Ambient temperature; | 53% |
-
-
201230-82-2
carbon monoxide

-
-
766-77-8
Dimethylphenylsilane

-
-
68452-41-5
N,N-dibenzylpropargylamine

-
A
-
5272-18-4
dimethylphenylsilanol

-
B
-
56-33-7
1,1,3,3-tetramethyl-1,3-diphenyldisiloxane

-
C
-
144776-76-1
2-(dimethylphenyl)silylmethyl-2-propenal

-
D
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| dodecacarbonyltetrarhodium(0) In benzene at 100℃; under 14710.2 Torr; for 2h; Product distribution; other temperature, other substrates; | A n/a B n/a C 94% D n/a |
| dodecacarbonyltetrarhodium(0) In benzene at 100℃; under 14710.2 Torr; for 2h; | A n/a B n/a C 94% D n/a |

-
-
72210-07-2
N,N-dibenzyl-4-methylbenzenesulfonamide

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate; chloro-trimethyl-silane; magnesium In tetrahydrofuran at 50℃; for 12h; Inert atmosphere; | 94% |
| With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; samarium diiodide In tetrahydrofuran for 8h; Heating; | 84% |
| With sodium hydride In N,N-dimethyl acetamide at 60℃; for 5h; Inert atmosphere; | 73% |

| Conditions | Yield |
|---|---|
| With Fe3O4-supported (diisopropylamino)acetamide In dichloromethane at 25℃; | 94% |
| In nitrobenzene at 40℃; Rate constant; Mechanism; | |
| In methanol at 24.84℃; Mechanism; Kinetics; Thermodynamic data; Temperature; | |
| With tetrabutylammomium bromide; sodium hydroxide In water at 15℃; for 4h; Catalytic behavior; Solvent; Reagent/catalyst; | 75 %Chromat. |
| In methanol at 30℃; Kinetics; Temperature; |
-
-
137406-90-7
N-benzylidene<α-(benzotriazol-1-yl)benzyl>amine

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 0.5h; Ambient temperature; | 94% |
-
-
22014-91-1
3-(dibenzylamino)prop-1-ene

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With aminomethyl resin-supported N-propylbarbituric acid; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 40℃; | 93% |
| With 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); diisobutylaluminium hydride In toluene for 1h; Ambient temperature; | 82% |
| Grubbs catalyst first generation In toluene at 110℃; for 3.5h; | 77% |
| Grubbs catalyst first generation In toluene for 3.5h; Heating; | 77% |

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride hydrate; potassium carbonate; triphenylphosphine at 140℃; for 9h; Inert atmosphere; | 93% |
| With hydrogen; potassium carbonate In acetonitrile under 760.051 Torr; for 30h; Irradiation; Green chemistry; chemoselective reaction; |
-
-
100-46-9
benzylamine

-
-
100-51-6
benzyl alcohol

-
A
-
780-25-6
N-benzylidene benzylamine

-
B
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With Ir(PPh3)(PPEP*) In toluene for 24h; Reagent/catalyst; Reflux; Inert atmosphere; Schlenk technique; Glovebox; | A 93% B 6% |
| With C48H71IrN2P2 In toluene at 135℃; for 24h; Reagent/catalyst; Inert atmosphere; Schlenk technique; Glovebox; | A 8% B 92% |
| With gold-ceria nanoparticle In dodecane at 180℃; for 6h; Autoclave; Inert atmosphere; | A 15% B 84% |


| Conditions | Yield |
|---|---|
| With ammonium bromide; ethylenediamine at 80℃; for 10h; Microwave irradiation; Inert atmosphere; neat (no solvent); | 93% |
| With ammonium iodide; hydrazine hydrate In ethanol at 70℃; for 5h; Inert atmosphere; Sealed tube; Microwave irradiation; | 92% |
-
-
558-30-5
2-methyl-1,2-epoxypropane

-
-
103-49-1
dibenzylamine

-
-
344868-41-3
1-dibenzylamino-2-methyl-propan-2-ol

| Conditions | Yield |
|---|---|
| In ethanol at 50℃; for 72h; | 100% |
| With water | |
| With lithium bromide In methanol at 20 - 65℃; for 6h; | |
| With lithium bromide at 20 - 60℃; for 18h; | |
| With lithium bromide at 20 - 60℃; for 18h; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 24h; | 100% |
| With potassium carbonate In acetonitrile at 20℃; for 16h; | 100% |
| With sodium perborate In water at 20℃; for 6.5h; Green chemistry; | 92% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 14h; Inert atmosphere; | 100% |
| With triethylamine In dichloromethane at 20℃; | 60% |

| Conditions | Yield |
|---|---|
| In benzene Ambient temperature; | 100% |


| Conditions | Yield |
|---|---|
| In chloroform at 10 - 15℃; for 12h; | 100% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
103-49-1
dibenzylamine

-
-
94226-56-9
tert-butyl 2-(dibenzylamino)ethanoate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; ethanol for 4h; Heating; | 100% |
| In 1,4-dioxane; ethanol for 8h; Reflux; | 94% |
| In ethanol at 20℃; for 4h; | 90% |
| In 1,4-dioxane; ethanol for 5h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With bromine; triethylamine; sodium nitrite; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane; water | 100% |
| With [NO(1+)*18-crown-6*H(NO3)2(1-)] In dichloromethane at 20℃; for 0.0833333h; | 100% |
| With aluminium trichloride; silica gel; sodium nitrite In dichloromethane at 20℃; for 0.5h; Nitrosation; | 99% |

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| In acetonitrile Heating; | 100% |
-
-
167934-71-6
(2R)-N-(2-bromopropionyl)bornane-10,2-sultam

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| In acetonitrile Heating; | 100% |
-
-
27871-49-4
(S)-Methyl lactate

-
-
103-49-1
dibenzylamine

-
-
188798-80-3
methyl (R)-2-(N,N-dibenzylamino)propanoate

| Conditions | Yield |
|---|---|
| Stage #1: (S)-Methyl lactate With 2,6-dimethylpyridine; trifluoromethylsulfonic anhydride In dichloromethane at 0℃; for 0.25h; Stage #2: dibenzylamine In dichloromethane at 20℃; for 2h; | 100% |
| With 2,6-dimethylpyridine; trifluoromethylsulfonic anhydride 1.) CH2Cl2, 0 deg C, 15 min, 2.) room temperature, 2 h; Yield given. Multistep reaction; |
-
-
31562-43-3
tert-butylsulfinyl chloride

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1h; sulfinylation; | 100% |
| In dichloromethane at 0℃; for 3.33333h; | 98% |
-
-
286-20-4
cyclohexene oxide

-
-
103-49-1
dibenzylamine

-
-
97807-82-4
trans-(+/-)-2-(dibenzylamino)cyclohexanol

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate In dichloromethane at 20℃; | 100% |
-
-
106-89-8
epichlorohydrin

-
-
103-49-1
dibenzylamine

-
-
99931-16-5
1-[bis(phenylmethyl)amino]-3-chloro-2-propanol

| Conditions | Yield |
|---|---|
| at 90℃; Inert atmosphere; | 100% |
| With calcium(II) trifluoromethanesulfonate In acetonitrile at 20℃; for 5h; | 91% |
| In methanol for 16h; | 90% |
-
-
917-92-0
2,2-dimethyl-3-butyne

-
-
201230-82-2
carbon monoxide

-
-
103-49-1
dibenzylamine

-
-
252667-35-9
(E)-4,4-dimethyl-N,N-bis(phenylmethyl)-2-pentenamide

| Conditions | Yield |
|---|---|
| Stage #1: 3,3-Dimethylbut-1-yne; carbon monoxide With quinoline; tri(2-furyl)germane; tris(2,4-di-tert-butylphenyl)phosphite; bis(η3-allyl-μ-chloropalladium(II)) In toluene at 20℃; under 760.051 Torr; for 5.5h; Stage #2: dibenzylamine; dmap In toluene at 70℃; for 6h; Further stages.; | 100% |

-
-
17392-83-5
(R)-Methyl lactate

-
-
103-49-1
dibenzylamine

-
-
87281-16-1
2-dibenzylamino-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (R)-Methyl lactate With 2,6-dimethylpyridine; trifluoromethylsulfonic anhydride In dichloromethane at 0℃; for 0.25h; Stage #2: dibenzylamine In dichloromethane at 20℃; for 2h; | 100% |
-
-
103-49-1
dibenzylamine

-
-
274694-17-6
(dibenzylamido)phosphoric dichloride

| Conditions | Yield |
|---|---|
| With trichlorophosphate In diethyl ether at 0℃; for 4h; | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
103-49-1
dibenzylamine

-
-
203866-88-0
dibenzyl-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.75h; | 100% |
| With perchloric acid at 30 - 35℃; for 0.166667h; | 100% |
| With guanidine hydrochloride In ethanol at 35 - 40℃; for 0.0166667h; | 100% |

| Conditions | Yield |
|---|---|
| chloro(1,5-cyclooctadiene)rhodium(I) dimer In 1,4-dioxane; ethanol at 120℃; under 37503 Torr; for 65h; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: isobutyraldehyde; dibenzylamine With chloro-trimethyl-silane; triethylamine-borane; lithium perchlorate In diethyl ether at 20℃; for 0.333333h; Stage #2: With triethylamine-borane; lithium perchlorate In diethyl ether at 20℃; for 1h; | 100% |
-
-
816444-14-1
tert-butyl (RS)-3-benzyl-cyclopent-1-enecarboxylate

-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| Stage #1: dibenzylamine With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: tert-butyl (RS)-3-benzyl-cyclopent-1-enecarboxylate In tetrahydrofuran; hexane at -78℃; for 3h; Stage #3: With potassium tert-butylate In hexane; tert-butyl alcohol for 3h; Heating; | 100% |
-
-
98-61-3
4-iodobenzenesulfonyl chloride

-
-
103-49-1
dibenzylamine

-
-
428486-70-8
N,N-dibenzyl-4-iodobenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 80℃; under 6723.1 Torr; for 0.111667h; | 100% |
| With triethylamine In dichloromethane at 20℃; for 16h; Inert atmosphere; | 92% |
| With triethylamine In dichloromethane at 20℃; for 24h; Inert atmosphere; | 89% |
-
-
103-49-1
dibenzylamine

-
-
816444-23-2
tert-butyl (RS)-3-ethylcyclopentene-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: dibenzylamine With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: tert-butyl (RS)-3-ethylcyclopentene-1-carboxylate In tetrahydrofuran; hexane at -78℃; for 3h; Stage #3: With potassium tert-butylate In hexane; tert-butyl alcohol for 3h; Heating; | 100% |
-
-
103-49-1
dibenzylamine

| Conditions | Yield |
|---|---|
| Stage #1: dibenzylamine With trimethylaluminum In toluene at 20℃; for 1h; Stage #2: 2'-trifluoromethanesulfonyloxy-[1,1']binaphthalenyl-2-carboxylic acid methyl ester In toluene for 3h; Heating; Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at -78 - 20℃; for 1.33333h; | 100% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at -78 - 20℃; for 1.33333h; | 100% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0℃; for 2h; | 83% |
-
-
23056-33-9
2-chloro-4-methyl-5-nitro-pyridine

-
-
103-49-1
dibenzylamine

-
-
867034-18-2
2-(dibenzylamino)-5-nitro-4-methylpyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-4-methyl-5-nitro-pyridine; dibenzylamine With sodium carbonate In toluene for 36h; Heating / reflux; Stage #2: With hydrogenchloride In dichloromethane; water | 100% |

| Conditions | Yield |
|---|---|
| 100% | |
| 100% |

Dibenzylamine Specification
The Dibenzylamine, with CAS registry number of 103-49-1, is also known as Benzenemethanamine,N-(phenylmethyl)-. The IUPAC name is N-Benzyl-1-phenylmethanamine. It belongs to product categories of Pharmaceutical Intermediates; Amines; C11 to C38; Nitrogen Compounds. Its EINECS registry number is 203-117-7. In addition, the formula is C14H15N and the molecular weight is 197.28. This chemical is a colorless to light yellow liquid that soluble in alcohol and ether, insoluble in water. Besides, it should be sealed in ventilated, cool place away from fire, heat, oxidants and even acids. This chemical is used as intermediates in organic synthesis and used for determination of cobalt, iron and cyanates.
Physical properties about Dibenzylamine are: (1)ACD/LogP: 3.42; (2)ACD/LogD (pH 5.5): 0.55; (3)ACD/LogD (pH 7.4): 2.05; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 9.98; (6)ACD/KOC (pH 5.5): 2.32; (7)ACD/KOC (pH 7.4): 73.67; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 4; (11)Index of Refraction: 1.58; (12)Molar Refractivity: 63.88 cm3; (13)Molar Volume: 191.7 cm3; (14)Surface Tension: 40.5 dyne/cm; (15)Density: 1.028 g/cm3; (16)Flash Point: 143.3 °C; (17)Enthalpy of Vaporization: 54 kJ/mol; (18)Boiling Point: 300 °C at 760 mmHg; (19)Vapour Pressure: 0.00115 mmHg at 25 °C.
Preparation of Dibenzylamine: it is prepared by reaction of benzaldehyde with benzylamine. The reaction needs reagent benzyltriphenylphosphonium borohydride and solvent methanol at the temperature of 20 °C for 20 minutes. The yield is about 98%.
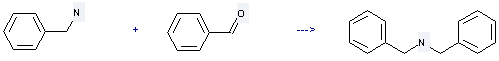
Uses of Dibenzylamine: it is used to produce dibenzyl-nitroso-amine. The reaction occurs with reagent cross-linked polyvinylpyrrolidone·N2O4 and solvent CH2Cl2 at 20 °C for 5 minutes. The yield is about 95%.

When you are using this chemical, please be cautious about it as the following:
As a chemical, it is irritating to eyes and skin. This chemical is harmful if swallowed and also is harmful to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. What's more, it causes burns. When using it, you should wear suitable protective clothing, gloves and eye/face protection. You must avoid releasing it to the environment. If contact with eyes accidently, you must rinse immediately with plenty of water and seek medical advice. In case of accident or if you feel unwell, you should seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C1=CC=C(C=C1)CNCC2=CC=CC=C2
(2) InChI: InChI=1S/C14H15N/c1-3-7-13(8-4-1)11-15-12-14-9-5-2-6-10-14/h1-10,15H,11-12H2
(3)InChIKey: BWLUMTFWVZZZND-UHFFFAOYSA-N
Related Products
- Dibenzylamine
- 1034912-28-1
- 10349-38-9
- 10349-57-2
- 103496-86-2
- 10350-10-4
- 103-50-4
- 103505-49-3
- 103505-54-0
- 10351-06-1
- 10351-19-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View