-
Name
Dichlorodifluoromethane
- EINECS 200-893-9
- CAS No. 75-71-8
- Article Data195
- CAS DataBase
- Density 1.535 g/cm3
- Solubility Water: 0.028 g/100 mL
- Melting Point -157.7 °C
- Formula CCl2F2
- Boiling Point -29.8 °C
- Molecular Weight 120.914
- Flash Point 11 °C
- Transport Information UN 1230 3/PG 2
- Appearance colourless odourless gas
- Safety 23-24/25-59-61-45-24-16-7-36/37
- Risk Codes 20-59-23/25-11-39/23/24/25-23/24/25
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn,
Xn, N,
N, T,
T, F
F
- Synonyms Algofrene type 2;CCRIS 3501;CFC-12;Chlorofluorocarbon 12;Diclorodifluometano;Dwuchlorodwufluorometan;Dymel 12;Electro-CF 12;Eskimon 12;F 12;FCC 12;FKW 12;Fluorocarbon-12;UNII-OFM06SG1KO;
- PSA 0.00000
- LogP 2.01430
Synthetic route

| Conditions | Yield |
|---|---|
| With ClSO3F for 3h; Ambient temperature; | 85% |
-
-
127-21-9
1,3-dichloro-1,1,3,3-tetrafluoro-propan-2-one

-
A
-
75-71-8
Dichlorodifluoromethane

-
B
-
354-27-8
chlorodifluoroacetyl fluoride

-
C
-
152239-91-3
C3Cl3F5O

| Conditions | Yield |
|---|---|
| With chlorine monofluoride; cesium fluoride 1.) -140 degC to -65 degC over 6 h, 2.) -65 degC, 4 h; | A n/a B n/a C 80% |

-
-
2154-59-8
difluoro-methylene

-
A
-
75-72-9
chlorotrifluoromethane

-
B
-
75-71-8
Dichlorodifluoromethane

-
C
-
75-69-4
trichlorofluoromethane

| Conditions | Yield |
|---|---|
| With chlorine | A 20% B 70% C 10% |
| With Cl2 | A 20% B 70% C 10% |

-
-
75-44-5
phosgene

-
-
7664-39-3
hydrogen fluoride

-
-
7782-50-5
chlorine

-
A
-
75-73-0
carbon tetrafluoride

-
B
-
75-72-9
chlorotrifluoromethane

-
C
-
75-71-8
Dichlorodifluoromethane

| Conditions | Yield |
|---|---|
| With catalyst : charcoal heating in autoclave, 425°C, 17 h, charcoal impregnated with FeCl3; | A 4% B 57% C 14% |

-
-
7791-16-4
antimony dichloride trifluoride

-
-
421-56-7
difluorochloro nitromethane

-
A
-
75-72-9
chlorotrifluoromethane

-
B
-
75-71-8
Dichlorodifluoromethane

| Conditions | Yield |
|---|---|
| at 100°C 6 h; | A 53% B 8% |
| at 100°C 6 h; | A 53% B 8% |

-
-
75-44-5
phosgene

-
-
7664-39-3
hydrogen fluoride

-
-
7782-50-5
chlorine

-
A
-
56-23-5
tetrachloromethane

-
B
-
75-72-9
chlorotrifluoromethane

-
C
-
75-71-8
Dichlorodifluoromethane

-
D
-
75-69-4
trichlorofluoromethane

| Conditions | Yield |
|---|---|
| With catalyst : charcoal heating in autoclave, 350°C, 6 h, charcoal impregnated with FeCl3; | A 7% B 13% C 47% D 7% |

-
-
34557-54-5
methane

-
-
75-71-8
Dichlorodifluoromethane

| Conditions | Yield |
|---|---|
| With chromium fluoride; hydrogen fluoride; chlorine; pyrographite at 275 - 340℃; | |
| With calcium fluoride; sulfur trioxide; calcium chloride at 350 - 400℃; |

| Conditions | Yield |
|---|---|
| With chlorine at 350℃; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride; antimony(III) fluoride Einw. von Chlor auf das entstandene Methylenfluorid; |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; lithium fluoride at -2℃; Electrolysis; |

| Conditions | Yield |
|---|---|
| With chlorine at 750 - 950℃; |

| Conditions | Yield |
|---|---|
| With chlorine |

| Conditions | Yield |
|---|---|
| at 440 - 745℃; under 400 Torr; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride | |
| With aluminum tri-bromide | |
| With hydrogen fluoride; antimonypentachloride; chlorine at 75℃; Kinetics; Further Variations:; Temperatures; time; |

| Conditions | Yield |
|---|---|
| With chlorine at 800℃; |
-
-
76-14-2
1,2-dichloro-1,1,2,2-tetrafluoroethane

-
A
-
116-14-3
polytetrafluoroethylene

-
B
-
75-46-7
trifluoromethan

-
C
-
75-71-8
Dichlorodifluoromethane

| Conditions | Yield |
|---|---|
| at 880℃; |

-
-
56-23-5
tetrachloromethane

-
A
-
75-72-9
chlorotrifluoromethane

-
B
-
75-71-8
Dichlorodifluoromethane

-
C
-
75-69-4
trichlorofluoromethane

| Conditions | Yield |
|---|---|
| With chlorine pentafluoride at 0℃; for 1.5h; Product distribution; |


| Conditions | Yield |
|---|---|
| With chlorine Irradiation; |
-
-
75-09-2
dichloromethane

-
A
-
75-10-5
Difluoromethane

-
B
-
593-70-4
R32

-
C
-
75-45-6
Chlorodifluoromethane

-
D
-
75-43-4
Dichlorofluoromethane

-
E
-
75-71-8
Dichlorodifluoromethane

-
F
-
75-69-4
trichlorofluoromethane

| Conditions | Yield |
|---|---|
| With xenon difluoride for 48h; Ambient temperature; other halogenocarbons; var. reaction time; |

-
-
75-72-9
chlorotrifluoromethane

-
A
-
56-23-5
tetrachloromethane

-
B
-
75-73-0
carbon tetrafluoride

-
C
-
75-71-8
Dichlorodifluoromethane

-
D
-
75-69-4
trichlorofluoromethane

| Conditions | Yield |
|---|---|
| ruby at 450℃; for 3h; Yield given; |


| Conditions | Yield |
|---|---|
| aluminum oxide; chromium(III) oxide at 400℃; Product distribution; further catalysts, further temp.; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride In neat (no solvent) reaction on heating for 3 hours to 160°C;; | 99.5% |
| In neat (no solvent) fluorination in presence of catalyst at higher temp.;; | |
| In neat (no solvent) fluorination in presence of catalyst at higher temp.;; |
-
-
75-71-8
Dichlorodifluoromethane

-
-
371-77-7
N,N-Bis(trifluoromethyl)amine

-
-
7739-47-1
1,1-dibromotrifluoro-2-azapropene

| Conditions | Yield |
|---|---|
| With boron tribromide at 100℃; for 16h; | 93% |
-
-
75-71-8
Dichlorodifluoromethane

| Conditions | Yield |
|---|---|
| In water at 20℃; | 90% |

| Conditions | Yield |
|---|---|
| In various solvent(s) for 24h; Ambient temperature; | 88% |
-
-
75-71-8
Dichlorodifluoromethane

-
-
24677-78-9
2,3-dihydroxybenzaldehyde

-
-
119895-68-0
2,2-difluoro-1,3-benzodioxol-4-yl-carbaldehyde

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide In water at 60℃; for 5h; Solvent; Temperature; | 88% |
| With sodium hydroxide In acetone at -35 - 80℃; for 15h; Solvent; Reagent/catalyst; Temperature; Sealed tube; | 85.6% |

| Conditions | Yield |
|---|---|
| With sodium dithionite; sodium hydrogencarbonate In dimethyl sulfoxide at 90℃; for 10h; | 86% |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride at 375℃; | 83.5% |
| bei der Einw. einer Hochspannungs-Entladung; | |
| With chromium fluoride; hydrogen fluoride | |
| With iron(III) trifluoride; hydrogen fluoride |
-
-
13997-90-5
chlorine fluorosulfate

-
-
75-71-8
Dichlorodifluoromethane

-
-
13036-75-4
fluorosulfonyl anhydride

| Conditions | Yield |
|---|---|
| With SbF5 In fluorosulphonic acid byproducts: CF2O; bubbling CF2Cl2 through a mixt. of ClOSO2F, SbF5, and HSO3F, <=40°C, 2 h; | 81.5% |
-
-
75-71-8
Dichlorodifluoromethane

-
-
89343-06-6
tris-iso-propylsilyl acetylene

-
-
284471-09-6
1-(triisopropylsilyl)-3-chloro-3-fluoropropyne

| Conditions | Yield |
|---|---|
| Stage #1: tris-iso-propylsilyl acetylene With n-butyllithium In tetrahydrofuran; hexane at 20℃; for 0.5h; Stage #2: Dichlorodifluoromethane In tetrahydrofuran; hexane at 0℃; for 1h; Further stages.; | 76% |

-
-
75-71-8
Dichlorodifluoromethane

-
A
-
40640-71-9
chlorodifluoromethyl cation

-
C
-
40640-70-8
fluorodichloromethyl(1+)

| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; the reactant and product ions are sampled; monitored by MAS; | A 31% B n/a C 69% |

-
-
75-71-8
Dichlorodifluoromethane

-
-
3111-52-2
potassium thiophenolate

-
A
-
85554-53-6
<(chlorodifluoromethyl)thio>benzene

-
B
-
1535-67-7
difluoromethyl phenyl sulfide

-
C
-
80351-59-3
difluorobis(phenylthio)methane

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide under 1520 Torr; Ambient temperature; | A 62% B 8% C 7% |
| In N,N-dimethyl-formamide under 2052 Torr; for 4h; | A 62% B 8% C 7% |

-
-
98-82-8
Isopropylbenzene

-
-
75-71-8
Dichlorodifluoromethane

-
A
-
934-53-2
cumenyl chloride

-
B
-
77116-52-0
(3-Chloro-3,3-difluoro-1-methylene-propyl)-benzene

-
C
-
77116-51-9
(2-Chloro-2,2-difluoro-1,1-dimethyl-ethyl)-benzene

-
D
-
98-83-9
isopropenylbenzene

| Conditions | Yield |
|---|---|
| at 200 - 250℃; Further byproducts given; | A 8 % Chromat. B 10 % Chromat. C 60% D 10 % Chromat. |
| at 200 - 250℃; Further byproducts given; | A 8 % Chromat. B 10 % Chromat. C 60% D 11 % Chromat. |

-
-
75-71-8
Dichlorodifluoromethane

-
-
82695-92-9
L-Homocysteine monosodium salt

-
-
126027-81-4
L-difluoromethionine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In ethanol | 56% |
-
-
75-71-8
Dichlorodifluoromethane

-
-
80793-25-5
3-(4-bromophenyl)propanal

-
-
1638192-46-7
1-bromo-4-(4,4-difluorobut-3-en-1-yl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: Dichlorodifluoromethane With triphenylphosphine In N,N-dimethyl-formamide at 20℃; for 0.666667h; Inert atmosphere; Heating; Stage #2: 3-(4-bromophenyl)propanal With zinc In N,N-dimethyl-formamide for 2h; Heating; | 54% |
-
-
75-71-8
Dichlorodifluoromethane

-
-
31367-69-8
potassium p-tolylthiolate

-
A
-
3447-50-5
<(difluoromethyl)thio>-4-methylbenzene

-
B
-
94169-13-8
<(chlorodifluoromethyl)thio>-4-methylbenzene

-
C
-
94169-14-9
difluorodi(4-methylthiophenoxy)methane

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide under 1520 Torr; Ambient temperature; | A 8% B 44% C 6% |
| In N,N-dimethyl-formamide under 2052 Torr; for 4h; | A 8% B 44% C 6% |

-
-
75-71-8
Dichlorodifluoromethane

-
-
2303-76-6, 118080-94-7
sodium diethyl phosphite

-
-
78715-58-9
Tetraethyl difluoromethylenediphosphonate

| Conditions | Yield |
|---|---|
| In toluene at -15℃; | 37% |
-
-
498-66-8
norborn-2-ene

-
-
75-71-8
Dichlorodifluoromethane

-
-
765-91-3, 2999-06-6, 29342-53-8, 36025-11-3, 67844-26-2, 67844-27-3, 130726-60-2
2-chlorobicyclo[2.2.1]heptane

-
-
77116-63-3
(1R,4S)-2-(Chloro-difluoro-methyl)-bicyclo[2.2.1]heptane

-
-
77116-64-4
(1S,4R)-2-Chloro-3-(chloro-difluoro-methyl)-bicyclo[2.2.1]heptane

| Conditions | Yield |
|---|---|
| at 200 - 250℃; | A 21% B 27% C 13 % Chromat. |

-
-
75-71-8
Dichlorodifluoromethane

-
-
108-98-5
thiophenol

-
A
-
85554-53-6
<(chlorodifluoromethyl)thio>benzene

-
B
-
1535-67-7
difluoromethyl phenyl sulfide

-
C
-
80351-59-3
difluorobis(phenylthio)methane

| Conditions | Yield |
|---|---|
| With sodium hydride 1.) DMF; 2.) irradiation, -40 deg C, 2h; Yield given. Multistep reaction; | A n/a B 6% C 22% |
| With sodium hydride 1.) DMF; 2.) irradiation, -40 deg C, 2h; Yield given. Multistep reaction; | A 6% B n/a C 22% |
| With sodium hydride 1.) DMF; 2.) irradiation, -40 deg C, 2h; Yield given. Multistep reaction; | A 6% B 6% C n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent) equilibrium over catalyst; equilibrium constant at 290-540°C;; | A n/a B 22% |

-
-
75-71-8
Dichlorodifluoromethane

-
B
-
13568-63-3
magnesium divanadate (V)

-
C
-
124-38-9
carbon dioxide

-
D
-
7727-18-6
vanadium(V) oxychloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) using a fixed-bed flow reactor system at atmospheric pressure; placing of of Mg3(VO4)2 in quartz reactor; heating at 723 K under 1% CCl2F2/He atmosphere with total flow rate of 30 ml/min for 5 h; monitoring by XRD and GC-TCD; | A n/a B 1% C n/a D n/a |

Dichlorodifluoromethane Consensus Reports
Dichlorodifluoromethane Standards and Recommendations
ACGIH TLV: TWA 1000 ppm; Not Classifiable as a Human Carcinogen
DFG MAK: 1000 ppm (5000 mg/m3)
DOT Classification: 2.2; Label: Nonflammable Gas
Dichlorodifluoromethane Analytical Methods
Dichlorodifluoromethane Specification
The Dichlorodifluoromethane with CAS registry number of 75-71-8 is also known as Methane,dichlorodifluoro-. The IUPAC name and product name are the same. It belongs to product categories of CFC; Refrigerants; Organics; DIA-DICChemical Class; Alpha Sort; D; DAlphabetic; Fluoro; Halogenated; Volatiles/ Semivolatiles. Its EINECS registry number is 200-893-9. In addition, the formula is CCl2F2 and the molecular weight is 120.91.
Physical properties about Dichlorodifluoromethane are: (1)ACD/LogP: 2.19; (2)ACD/LogD (pH 5.5): 2.19; (3)ACD/LogD (pH 7.4): 2.19; (4)ACD/BCF (pH 5.5): 27.09; (5)ACD/BCF (pH 7.4): 27.09; (6)ACD/KOC (pH 5.5): 369.15; (7)ACD/KOC (pH 7.4): 369.15; (8)Index of Refraction: 1.343; (9)Molar Refractivity: 16.67 cm3; (10)Molar Volume: 78.7 cm3; (11)Polarizability: 6.6×10-24cm3; (12)Surface Tension: 18.3 dyne/cm; (13)Density: 1.535 g/cm3; (14)Enthalpy of Vaporization: 21.48 kJ/mol.
Preparation of Dichlorodifluoromethane: it is prepared by reaction of carbon tetrachloride with hydro fluoride. The reaction needs catalyst antimony pentachloride at the temperature of -5 °C.
![]()
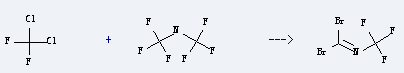
Uses of Dichlorodifluoromethane: it can be used as refrigerant, fire extinguishing agent, pesticide and spray. It is used to produce trifluormethyl-dibrom-methylenimin by reaction with bis-trifluoromethyl-amine. The reaction occurs with reagent BBr3 at 100 °C for 16 hours. The yield is about 93%.
When you are using this chemical, please be cautious about it. As a chemical, it is highly flammable and dangerous for the ozone layer. What's more, it is danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing and gloves. Avoid contact with skin and eyes and do not breathe gas/fumes/vapour/spray. Keep container tightly closed and away from sources of ignition. This material and its container must be disposed of as hazardous waste. In case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C(F)(F)(Cl)Cl
2. InChI: InChI=1S/CCl2F2/c2-1(3,4)5
3. InChIKey: PXBRQCKWGAHEHS-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LC50 | inhalation | 80pph/30M (800000ppm) | European Journal of Toxicology and Environmental Hygiene. Vol. 9, Pg. 385, 1976. | |
| human | TCLo | inhalation | 200000ppm/30M (200000ppm) | SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE LUNGS, THORAX, OR RESPIRATION: FIBROSING ALVEOLITIS LIVER: OTHER CHANGES | European Journal of Toxicology and Environmental Hygiene. Vol. 9, Pg. 385, 1976. |
| mouse | LC50 | inhalation | 3348gm/m3/3H (3348000mg/m3) | BEHAVIORAL: SLEEP BEHAVIORAL: EXCITEMENT BEHAVIORAL: TREMOR | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 28(6), Pg. 95, 1963. |
| rabbit | LC50 | inhalation | 80pph/30M (800000ppm) | European Journal of Toxicology and Environmental Hygiene. Vol. 9, Pg. 385, 1976. | |
| rat | LC | inhalation | > 80pph/4H (800000ppm) | National Technical Information Service. Vol. OTS0520424, | |
| rat | LD | oral | > 5600ug/kg (5.6mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(3), Pg. 73, 1987. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View