-
Name
Dichloromonofluoromethane
- EINECS 200-869-8
- CAS No. 75-43-4
- Article Data57
- CAS DataBase
- Density 1.397 g/cm3
- Solubility Slightly soluble in water
- Melting Point -135 °C
- Formula CHCl2F
- Boiling Point 8.9 °C at 760 mmHg
- Molecular Weight 102.923
- Flash Point 130-132°C/3mm
- Transport Information
- Appearance COLOURLESS GAS OR COMPRESSED LIQUEFIED GAS, WITH CHARACTERISTIC ODOUR.
- Safety 59
- Risk Codes 59
-
Molecular Structure
-
Hazard Symbols
 N
N
- Synonyms AF 22;AF22 (fluorocarbon);Algofrene Type 5;Arcton 7;CFC 21;Dichlorofluoromethane;Dichloromonofluoromethane;F 21;F 21 (fluorocarbon);FC 21;Fluorocarbon-21;Fluorodichloromethane;Freon 21;Genetron 21;HCFC 21;Monofluorodichloromethane;R 21;R 21 (refrigerant);
- PSA 0.00000
- LogP 1.71710
Synthetic route
-
-
87375-48-2
1,1-dichloroperfluoro-2-butanone

-
A
-
75-43-4
Dichlorofluoromethane

-
B
-
141-53-7
sodium formate

-
C
-
378-77-8
sodium pentafluoropropionate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Product distribution; | A 33.5% B n/a C 84.5% |


| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide In N,N-dimethyl-formamide at 20℃; for 0.5h; | 20% |
| With ruthenium(II) bis(triphenylphosphine) dichloride; hydrogen In ethanol; toluene at 25℃; for 12h; Product distribution; also carbon tetrachloride; also in the presence of RuCl2(dppe)2 or RuHCl(PPh3)2; other solvents; var. time and temp.; | |
| titanium pH=7; Kinetics; | |
| With formate; titanium(IV) oxide In various solvent(s) at 23℃; for 1h; pH=5.9; Kinetics; Further Variations:; pH-values; Irradiation; |

| Conditions | Yield |
|---|---|
| With uranium hexafluoride | |
| With hydrogen fluoride; lithium fluoride at 0℃; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride; antimony(III) fluoride unter Druck; | |
| With hydrogen fluoride; antimonypentachloride; antimony(III) chloride | |
| With hydrogen fluoride; titanium(IV) fluoride at 127℃; |
-
-
75-09-2
dichloromethane

-
A
-
75-10-5
Difluoromethane

-
B
-
593-70-4
R32

-
C
-
75-45-6
Chlorodifluoromethane

-
D
-
75-43-4
Dichlorofluoromethane

-
E
-
75-71-8
Dichlorodifluoromethane

-
F
-
75-69-4
trichlorofluoromethane

| Conditions | Yield |
|---|---|
| With xenon difluoride for 48h; Ambient temperature; other halogenocarbons; var. reaction time; |

-
-
75-45-6
Chlorodifluoromethane

-
A
-
75-46-7
trifluoromethan

-
B
-
75-43-4
Dichlorofluoromethane

-
C
-
67-66-3
chloroform

| Conditions | Yield |
|---|---|
| zinc aluminate at 300℃; Product distribution; other temperature; |

-
-
67-66-3
chloroform

-
A
-
75-46-7
trifluoromethan

-
B
-
75-45-6
Chlorodifluoromethane

-
C
-
75-43-4
Dichlorofluoromethane

| Conditions | Yield |
|---|---|
| With antimony tetrachloride fluoride at 100℃; Kinetics; Product distribution; kinetic behaviour of different fluorinated antimony compound in fluorination (SbCl4F, SbCl5 + SbF3Cl2, SbCl5 + SbF5, SbCl5 + SbF3) at different reaction times; | |
| With antimony tetrachloride fluoride at 100℃; under 7600 Torr; Kinetics; Product distribution; Equilibrium constant; kinetic at three different temperatures (85, 100, 115 deg C) and at different pressures ( 7.3, 8.0, 10.0, 10.5, 13.0, 13.8 atm); | |
| With hydrogen fluoride; antimonypentachloride at 70 - 90℃; under 10746.4 - 10953.3 Torr; Product distribution / selectivity; Heating / reflux; |

-
-
70393-08-7
(dichloro-fluoro-methyl)-tris-dimethylamino-phosphonium; chloride

-
A
-
75-43-4
Dichlorofluoromethane

-
B
-
75-69-4
trichlorofluoromethane

-
C
-
353-58-2
bromodichlorofluoromethane

-
D
-
353-55-9
dibromochlorofluoromethane

| Conditions | Yield |
|---|---|
| With potassium fluoride; bromine In various solvent(s) | A 25 % Spectr. B 15 % Spectr. C 42 % Spectr. D 5 % Spectr. |

-
-
75-09-2
dichloromethane

-
A
-
75-10-5
Difluoromethane

-
B
-
593-70-4
R32

-
C
-
75-45-6
Chlorodifluoromethane

-
D
-
75-43-4
Dichlorofluoromethane

-
E
-
75-71-8
Dichlorodifluoromethane

| Conditions | Yield |
|---|---|
| With osmium pentafluoride oxide Product distribution; other transition-metal oxide fluorides; |

-
-
79-51-6
1,1,3,3-tetrachloro-1,3-difluoro-2-propanone

-
-
67-64-1
acetone

-
A
-
75-43-4
Dichlorofluoromethane

-
B
-
1717-00-6
HCFC-141b

-
C
-
75-69-4
trichlorofluoromethane

-
D
-
2317-91-1
1-chloro-1-fluoroethane

-
E
-
75-35-4
1,1-Dichloroethylene

-
F
-
76-12-0
CFC-112a

| Conditions | Yield |
|---|---|
| With propene; sulphur hexafluoride for 0.5h; Rate constant; Ambient temperature; Irradiation; also with acetone-d6; |


| Conditions | Yield |
|---|---|
| With (PPh3)2Pd(Ph)F; bis(triphenylphosphine)iminium chloride for 24h; | A 1 % Spectr. B n/a |
| With (PPh3)2Pd(Ph)F; bis(triphenylphosphine)iminium chloride for 24h; Yield given; |

| Conditions | Yield |
|---|---|
| at 100℃; |


| Conditions | Yield |
|---|---|
| Electrolysis; |


-
-
75-43-4
Dichlorofluoromethane

| Conditions | Yield |
|---|---|
| With chlorine |
-
-
67-66-3
chloroform

-
-
7647-18-9
antimonypentachloride

-
-
7783-56-4
antimony(III) fluoride

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
75-43-4
Dichlorofluoromethane

-
-
67-66-3
chloroform

-
-
7664-39-3
hydrogen fluoride

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
75-43-4
Dichlorofluoromethane

-
-
67-66-3
chloroform

-
-
7664-39-3
hydrogen fluoride

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
75-43-4
Dichlorofluoromethane

-
-
110-71-4
1,2-dimethoxyethane

-
-
420-48-4
fluorodichloroiodomethane

-
A
-
75-43-4
Dichlorofluoromethane

-
B
-
76-12-0
CFC-112a

-
-
79-52-7
1,1,3-trichloro-1,3,3-trifluoroacetone

-
-
71-43-2
benzene

-
A
-
75-43-4
Dichlorofluoromethane

-
B
-
76-04-0
2-chloro-2,2-difluoroacetic acid

-
-
354-19-8
dichlorofluoroacetic acid

-
-
7732-18-5
water

-
A
-
64-18-6
formic acid

-
B
-
75-43-4
Dichlorofluoromethane

-
C
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

| Conditions | Yield |
|---|---|
| at 71 - 95℃; Rate constant; |

-
-
75-09-2
dichloromethane

-
A
-
593-70-4
R32

-
B
-
75-45-6
Chlorodifluoromethane

-
C
-
75-43-4
Dichlorofluoromethane

| Conditions | Yield |
|---|---|
| With rhenium(VII) fluoride for 4h; Product distribution; other transition metal fluorides; |

-
-
75-69-4
trichlorofluoromethane

-
A
-
593-70-4
R32

-
B
-
75-09-2
dichloromethane

-
C
-
75-43-4
Dichlorofluoromethane

-
D
-
67-66-3
chloroform

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; mechanism discussed;; |

-
-
75-46-7
trifluoromethan

-
-
67-66-3
chloroform

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
75-43-4
Dichlorofluoromethane

| Conditions | Yield |
|---|---|
| aluminum(III) fluoride at 240℃; for 0.00858333h; | |
| aluminum(III) fluoride at 160℃; for 0.00111111h; |

-
-
75-46-7
trifluoromethan

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
75-43-4
Dichlorofluoromethane

-
C
-
67-66-3
chloroform

| Conditions | Yield |
|---|---|
| With dichloromethane; chlorine at 250℃; for 0.00138889h; Concentration; Temperature; Reagent/catalyst; | A 6.9 %Chromat. B 7.2 %Chromat. C 23.7 %Chromat. |

-
-
111-34-2
-butyl vinyl ether

-
-
75-43-4
Dichlorofluoromethane

-
-
95241-05-7
2-n-Butoxy-1-chloro-1-fluorocyclopropane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 40℃; for 12h; Inert atmosphere; | 96% |
| With sodium hydroxide; tetraethylammonium bromide In dichloromethane at 5℃; for 6h; | 79% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In hexane at -10℃; | 92% |
-
-
543-75-9
1,4-dioxene

-
-
75-43-4
Dichlorofluoromethane

-
-
40623-35-6, 40623-36-7, 120201-74-3
7-chloro-7-fluoro-2,5-dioxabicyclo<4.1.0>heptane

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane at 20℃; for 14h; | 91% |
-
-
75-43-4
Dichlorofluoromethane

-
-
115-11-7
isobutene

-
-
1891-96-9
1-chloro-1-fluoro-2,2-dimethylcyclopropane

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; potassium hydroxide In dichloromethane at 0 - 20℃; | 88% |
| With N-benzyl-N,N,N-triethylammonium chloride; potassium hydroxide In water at 0℃; for 4h; Cooling with ice; | 87% |
| With N-benzyl-N,N,N-triethylammonium chloride; potassium hydroxide In dichloromethane; water at 0℃; for 4h; | 87% |
-
-
177698-19-0
Beta-pinene

-
-
75-43-4
Dichlorofluoromethane

-
-
135654-99-8, 135684-70-7
2'-chloro-2'-fluoro-6,6-dimethylbicyclo<3.1.1>heptane-2-spiro-1'-cyclopropane

| Conditions | Yield |
|---|---|
| With potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride for 10h; ultrasonicator; | 85% |
-
-
75-43-4
Dichlorofluoromethane

-
-
78-79-5
isoprene

-
-
17725-43-8
1-chloro-1-fluoro-2-methyl-2-vinylcyclopropane

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane; water at 10℃; | 85% |
| With potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane; water at -10 - -5℃; for 8h; | 78% |
| With N-benzyl-N,N,N-triethylammonium chloride; potassium hydroxide In dichloromethane at -5 - 0℃; for 9h; |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In hexane at -10℃; | 83% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In hexane at -10℃; | 83% |
-
-
563-79-1
2,3-Dimethyl-2-butene

-
-
75-43-4
Dichlorofluoromethane

-
-
1727-63-5
1-chloro-1-fluoro-2,2,3,3-tetramethylcyclopropane

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; potassium hydroxide In dichloromethane; water at 0℃; for 4h; | 81% |
| With n-butyllithium In hexane | |
| With n-butyllithium |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether for 1h; | 80% |
-
-
75-43-4
Dichlorofluoromethane

-
-
95311-30-1, 95311-49-2
2-(Chloro-fluoro-methyl)-2-isopropenyl-malonic acid tert-butyl ester ethyl ester

-
-
95311-35-6
2-Fluoromethyl-2-isopropenyl-malonic acid tert-butyl ester ethyl ester

| Conditions | Yield |
|---|---|
| With tri-n-butyl-tin hydride; 2,2'-azobis(isobutyronitrile) In benzene for 2h; Heating; | 80% |
-
-
75-43-4
Dichlorofluoromethane

-
-
69555-16-4
ethyl 2-(diphenylmethyleneamino)propanoate

| Conditions | Yield |
|---|---|
| With potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride; water In dichloromethane at 7 - 8℃; for 4.33333h; Cycloaddition; | 79% |
-
-
75-43-4
Dichlorofluoromethane

-
-
79-35-6
1,1'-dichloro-2,2'-difluoroethene

-
A
-
422-52-6
1,1,3,3-tetrachloro-1,2,2-trifluoropropane

-
B
-
422-35-5
1,1,2,2-tetrachloro-3,3,3-trifluoropropane

-
C
-
422-50-4
1,1,1,3-tetrachloro-2,2,3-trifluoropropane

-
D
-
144909-54-6
1,2,2,3-tetrachloro-1,1,3-trifluoropropane

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 0℃; for 10h; | A 78.5% B 5.3% C n/a D 16.1% |

-
-
75-43-4
Dichlorofluoromethane

-
-
102069-99-8
1-ethoxy-1-phenoxy ethylene

| Conditions | Yield |
|---|---|
| With potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane; water at 0℃; for 2h; | 76% |
-
-
75-43-4
Dichlorofluoromethane

-
-
40012-82-6
N-(2,2-diphenylvinylidene)-4-methoxyaniline

-
-
134920-50-6
C22H18FNO2

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In hexane at -10℃; | 76% |
-
-
75-43-4
Dichlorofluoromethane

-
-
89343-06-6
tris-iso-propylsilyl acetylene

-
-
284471-09-6
1-(triisopropylsilyl)-3-chloro-3-fluoropropyne

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 1h; Alkylation; | 76% |
-
-
75-43-4
Dichlorofluoromethane

-
-
124746-03-8
1-(3-Methyl-but-3-en-1-ynyl)-adamantane

-
A
-
124746-13-0
1-(1-adamantylethinyl)-2-chloro-2-fluoro-1-methyl-cyclopropane

-
B
-
124746-17-4
1-adamantyl-2-(2-chloro-2-fluoro-1-methylcyclopropyl)-cyclopropenone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In ethanol; dichloromethane for 24h; Ambient temperature; | A 75% B 3% |

-
-
75-43-4
Dichlorofluoromethane

-
-
536-74-3
phenylacetylene

-
-
108234-26-0
1-chloro-1-fluoro-3-phenylprop-2-yne

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at -100 - -70℃; | 75% |
-
-
75-43-4
Dichlorofluoromethane

-
-
73046-16-9
1,3-di-tert-butylcyclopentadiene

-
-
65130-67-8
1,3-di-tert-butyl-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane for 4h; Ambient temperature; | 75% |

| Conditions | Yield |
|---|---|
| With Aliquat 336; methoxybenzene; potassium hydroxide In water at 20 - 30℃; for 3h; Reagent/catalyst; | 74.8% |
| With tetrabutylammomium bromide; potassium hydroxide In hexane; water at 5 - 10℃; | 27 g |
-
-
3725-05-1
2,5-dimethylhexa-1,5-dien-3-yne

-
-
75-43-4
Dichlorofluoromethane

-
-
124746-14-1
bis-(2-chloro-2-fluoro-1-methylcyclopropyl)acetylene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In ethanol; dichloromethane for 24h; Ambient temperature; | 73% |
-
-
624-64-6
trans-2-Butene

-
-
75-43-4
Dichlorofluoromethane

-
-
16496-06-3, 16496-07-4, 56586-48-2
1-Chlor-1-fluor-2,3-dimethylcyclopropan

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; potassium hydroxide In dichloromethane at 0 - 20℃; | 73% |

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; potassium hydroxide In water at 0℃; for 4h; Cooling with ice; | 73% |
| With N-benzyl-N,N,N-triethylammonium chloride; potassium hydroxide In dichloromethane; water at 0℃; for 4h; | 73% |
-
-
75-43-4
Dichlorofluoromethane

-
-
563-47-3
3-Chloro-2-methylpropene

-
-
40841-57-4
1-Chlor-2-chlormethyl-1-fluor-2-methylcyclopropan

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether at 0℃; for 1h; | 72% |
| With potassium hydroxide; benzyltrimethylammonium chloride | |
| With perhydrodibenzo-18-crown-6; potassium hydroxide |
-
-
57188-99-5
3-butyn-2-yl tetrahydropyranyl ether

-
-
75-43-4
Dichlorofluoromethane

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at -100 - -70℃; | 70% |
-
-
75-43-4
Dichlorofluoromethane

-
-
53309-84-5
(E)-1-chloro-3-methylpent-2-ene

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether for 1h; | 70% |
-
-
75-43-4
Dichlorofluoromethane

-
-
6089-04-9
3-(tetrahydropyran-2'-yloxy)propyne

-
-
108268-38-8
2-(4-chloro-4-fluorobut-2-ynyloxy)-tetrahydro-2H-pyran

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at -100 - -70℃; | 70% |
| Stage #1: 3-(tetrahydropyran-2'-yloxy)propyne With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: Dichlorofluoromethane In tetrahydrofuran; hexane at -100 - -50℃; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; benzyltrimethylammonium chloride In water; benzene at 50℃; under 2206.5 - 3677.5 Torr; for 4h; | 70% |
-
-
75-43-4
Dichlorofluoromethane

-
-
503-60-6
3,3-dimethyl-allyl chloride

-
-
344323-87-1
1-Chloro-3-chlormethyl-1-fluor-2,2-dimethylcyclopropan

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether for 1h; | 70% |
Dichloromonofluoromethane Consensus Reports
Dichloromonofluoromethane Standards and Recommendations
ACGIH TLV: TWA 10 ppm
DFG MAK: 10 ppm (43 mg/m3)
DOT Classification: 2.2; Label: Nonflammable Gas
Dichloromonofluoromethane Analytical Methods
Dichloromonofluoromethane Specification
The Dichlorofluoromethane is an organic compound with the formula CHCl2F. The IUPAC name of this chemical is dichloro(fluoro)methane. With the CAS registry number 75-43-4, it is also named as methane, dichlorofluoro-. The product's categories are Refrigerants; Organics. Besides, it should be stored in a cool and ventilated place. It is used as a refrigerant.
Physical properties about Dichlorofluoromethane are: (1)ACD/LogP: 1.40; (2)ACD/LogD (pH 5.5): 1.4; (3)ACD/LogD (pH 7.4): 1.4; (4)ACD/BCF (pH 5.5): 6.83; (5)ACD/BCF (pH 7.4): 6.83; (6)ACD/KOC (pH 5.5): 137.75; (7)ACD/KOC (pH 7.4): 137.75; (8)Index of Refraction: 1.366; (9)Molar Refractivity: 16.5 cm3; (10)Molar Volume: 73.6 cm3; (11)Polarizability: 6.54×10-24cm3; (12)Surface Tension: 19.9 dyne/cm; (13)Density: 1.397 g/cm3; (14)Enthalpy of Vaporization: 24.65 kJ/mol; (15)Boiling Point: 8.9 °C at 760 mmHg; (16)Vapour Pressure: 1340 mmHg at 25°C.
Preparation: this chemical can be prepared by trichloro-fluoro-methane. This reaction will need reagent HMPT and solvent dimethylformamide. The reaction time is 30 min with reaction temperature of 20 °C. The yield is about 20%.

Uses of Dichlorofluoromethane: it can be used to produce 7-chlor-7-fluor-1-methylbicyclo[4.1.0]heptan . It will need reagent 55percent KOH, 18-crown-6 with reaction time of 1 hour. The yield is about 80%.
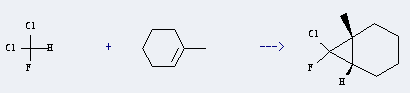
You can still convert the following datas into molecular structure:
(1)SMILES: ClC(Cl)F
(2)InChI: InChI=1/CHCl2F/c2-1(3)4/h1H
(3)InChIKey: UMNKXPULIDJLSU-UHFFFAOYAU
(4)Std. InChI: InChI=1S/CHCl2F/c2-1(3)4/h1H
(5)Std. InChIKey: UMNKXPULIDJLSU-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LCLo | inhalation | 10pph/1H (100000ppm) | Fluorine Chemistry Reviews. Vol. 1, Pg. 197, 1967. | |
| mouse | LC50 | inhalation | > 800gm/m3/2H (800000mg/m3) | BEHAVIORAL: TREMOR BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 20(11), Pg. 38, 1976. |
| rat | LC50 | inhalation | 49900ppm/4H (49900ppm) | "Documentation of the Threshold Limit Values and Biological Exposure Indices," 5th ed., Cincinnati, OH, American Conference of Governmental Industrial Hygienists, Inc., 1986Vol. 5, Pg. 187, 1986. |
Related Products
- Dichloromonofluoromethane
- 754-34-7
- 75434-70-7
- 7543-51-3
- 75436-40-7
- 75438-54-9
- 75438-57-2
- 75438-58-3
- 75444-69-8
- 75-44-5
- 7544-75-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View