-
Name
Chlorodifluoromethane
- EINECS 200-871-9
- CAS No. 75-45-6
- Article Data92
- CAS DataBase
- Density 1.277 g/cm3
- Solubility slightly soluble in water
- Melting Point -146 °C
- Formula CHClF2
- Boiling Point -40.8 °C
- Molecular Weight 86.4687
- Flash Point
- Transport Information UN 1018
- Appearance Colourless gas
- Safety 23-59
- Risk Codes 40-59
-
Molecular Structure
-
Hazard Symbols
 F
F
- Synonyms Algeon 22;Algofrene 22;Algofrene 6;Arcton 22;Arcton 4;CFC 22;Chlorodifluoromethane;Daiflon 22;Difluoromonochloromethane;Dymel 22;F 22 (halocarbon);FC 22;FC 22 (halocarbon);FKW 22;Flugene22;Forane 22;Freon 22;Frigen 22;Fron 22;HFA 22;Haltron 22;Isceon 22;Isotron 22;Khladon 22;Korfron 22;Propellant 22;Refrigerant R 22;Solkane 22;Ucon 22;
- PSA 0.00000
- LogP 1.44780
Synthetic route

-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| With 2,2,2-trifluoroethanol In N,N,N,N,N,N-hexamethylphosphoric triamide decompn. in the presence of CF3CH2OH at reflux temp., 12 h; | 100% |
| In N,N,N,N,N,N-hexamethylphosphoric triamide |
-
-
75-09-2
dichloromethane

-
-
7664-41-7
ammonia

-
A
-
74-90-8
hydrogen cyanide

-
B
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| nickel In neat (no solvent) steady-state flow reaction over Ni catalyst at 723 K; | A 98.3% B 0.6% |
| nickel In neat (no solvent) steady-state flow reaction over Ni catalyst at 673 K; | A 75.4% B 1.7% |

-
-
79-53-8
chloropentafluoroacetone

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
354-34-7
trifluoroacetyl fluoride

-
C
-
2925-22-6
2,2-difluoroacetyl fluoride

-
D
-
152239-89-9
C3HClF6O

| Conditions | Yield |
|---|---|
| With chlorine monofluoride; cesium fluoride 1.) -140 degC to -75 degC over 6 h, 2.) -75 degC, 2 h; Further byproducts given; | A n/a B n/a C n/a D 90% |

-
-
75-09-2
dichloromethane

-
-
7664-41-7
ammonia

-
A
-
74-90-8
hydrogen cyanide

-
B
-
75-10-5
Difluoromethane

-
C
-
75-45-6
Chlorodifluoromethane

-
D
-
124-38-9
carbon dioxide

-
E
-
201230-82-2
carbon monoxide

| Conditions | Yield |
|---|---|
| nickel titanate In neat (no solvent) steady-state flow reaction over NiTO3*6H2O catalyst at 773 K, further by-product is CFH3 (yield 0.3 %); | A 81.8% B 0.1% C 0.6% D 10.4% E 6.8% |
| nickel titanate In neat (no solvent) steady-state flow reaction over NiTO3*6H2O catalyst at 823 K, further by-product is CFH3 (yield 0.4 %); | A 66.2% B 0.2% C 1.4% D 12.9% E 18.8% |
| With catalyst: Pt/C In neat (no solvent) steady-state flow reaction over Pt/C catalyst at 821 K, a further by-product is CFH3 (yield 0.2 %); | A 64.6% B 0.3% C 0.2% D 0.6% E 0.8% |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; | 68% |
-
-
75-09-2
dichloromethane

-
-
7664-41-7
ammonia

-
A
-
74-90-8
hydrogen cyanide

-
B
-
593-53-3
Methyl fluoride

-
C
-
75-10-5
Difluoromethane

-
D
-
75-45-6
Chlorodifluoromethane

-
E
-
124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| With catalyst: Pt/C In neat (no solvent) steady-state flow reaction over Pt/C catalyst at 769 K; | A 53.4% B 0.3% C 0.5% D 7.6% E 0.5% |

-
-
1717-59-5
2,2-difluoro-2-(fluorosulfonyl)acetic acid

-
-
1005-30-7
sodium 4-iodo-benzoate

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
120608-87-9
4-Iodo-benzoic acid difluoromethyl ester

| Conditions | Yield |
|---|---|
| With potassium chloride In acetonitrile at 50℃; for 2.5h; | A n/a B 45% |

-
-
79-38-9
Chlorotrifluoroethylene

-
-
152239-89-9
C3HClF6O

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
354-34-7
trifluoroacetyl fluoride

-
C
-
2925-22-6
2,2-difluoroacetyl fluoride

| Conditions | Yield |
|---|---|
| In trichlorofluoromethane for 18h; -135 degC to 25 degC; Further byproducts given; | A n/a B n/a C n/a D 40% |


| Conditions | Yield |
|---|---|
| In ethanol decompn. with alc. KOH at 35°C in 48h;; | A 24% B n/a |
| In ethanol |

-
-
75-43-4
Dichlorofluoromethane

-
-
1188-14-3
Triethylgerman

-
A
-
75-10-5
Difluoromethane

-
B
-
75-09-2
dichloromethane

-
C
-
75-46-7
trifluoromethan

-
D
-
75-45-6
Chlorodifluoromethane

-
E
-
994-28-5
triethylchlorogermane

| Conditions | Yield |
|---|---|
| With ACF. Et3GeH at 25℃; for 72h; Schlenk technique; Inert atmosphere; | A n/a B n/a C 22% D 16% E n/a |

-
-
34557-54-5
methane

-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| With chromium fluoride; hydrogen fluoride; chlorine at 275 - 300℃; |

| Conditions | Yield |
|---|---|
| With chlorine |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; lithium fluoride Electrolysis; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride Siedetemperatur; |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; antimonypentachloride at 80℃; | |
| With calcium fluoride; silver fluoride at 250℃; | |
| With antimony(III) fluoride |

| Conditions | Yield |
|---|---|
| With hydrogen; platinum at 685℃; | |
| With ammonium persulfate; ammonium formate In N,N-dimethyl-formamide at 30 - 40℃; | |
| With hydrogen Heating; |
-
-
116-15-4
perfluoropropylene

-
A
-
116-14-3
polytetrafluoroethylene

-
B
-
75-45-6
Chlorodifluoromethane

-
C
-
76-16-4
Hexafluoroethane

| Conditions | Yield |
|---|---|
| Ambient temperature; Irradiation; also CF2HCl; |

-
-
75-09-2
dichloromethane

-
A
-
75-10-5
Difluoromethane

-
B
-
593-70-4
R32

-
C
-
75-45-6
Chlorodifluoromethane

-
D
-
75-43-4
Dichlorofluoromethane

-
E
-
75-71-8
Dichlorodifluoromethane

-
F
-
75-69-4
trichlorofluoromethane

| Conditions | Yield |
|---|---|
| With xenon difluoride for 48h; Ambient temperature; other halogenocarbons; var. reaction time; |

-
-
75-43-4
Dichlorofluoromethane

-
A
-
75-09-2
dichloromethane

-
B
-
75-45-6
Chlorodifluoromethane

-
C
-
67-66-3
chloroform

| Conditions | Yield |
|---|---|
| With silica gel at 149.9℃; for 6h; Product distribution; |


| Conditions | Yield |
|---|---|
| With antimony tetrachloride fluoride at 100℃; Kinetics; kinetic batch run; |
-
-
75-43-4
Dichlorofluoromethane

-
A
-
75-46-7
trifluoromethan

-
B
-
75-45-6
Chlorodifluoromethane

-
C
-
67-66-3
chloroform

| Conditions | Yield |
|---|---|
| zinc aluminate at 250℃; Product distribution; other temperature; |

-
-
67-66-3
chloroform

-
A
-
75-46-7
trifluoromethan

-
B
-
75-45-6
Chlorodifluoromethane

-
C
-
75-43-4
Dichlorofluoromethane

| Conditions | Yield |
|---|---|
| With antimony tetrachloride fluoride at 100℃; Kinetics; Product distribution; kinetic behaviour of different fluorinated antimony compound in fluorination (SbCl4F, SbCl5 + SbF3Cl2, SbCl5 + SbF5, SbCl5 + SbF3) at different reaction times; | |
| With antimony tetrachloride fluoride at 100℃; under 7600 Torr; Kinetics; Product distribution; Equilibrium constant; kinetic at three different temperatures (85, 100, 115 deg C) and at different pressures ( 7.3, 8.0, 10.0, 10.5, 13.0, 13.8 atm); | |
| With hydrogen fluoride; antimonypentachloride at 70 - 90℃; under 10746.4 - 10953.3 Torr; Product distribution / selectivity; Heating / reflux; |

-
-
75-71-8
Dichlorodifluoromethane

-
A
-
74-90-8
hydrogen cyanide

-
B
-
593-53-3
Methyl fluoride

-
C
-
75-10-5
Difluoromethane

-
D
-
75-45-6
Chlorodifluoromethane

-
E
-
124-38-9
carbon dioxide

-
F
-
201230-82-2
carbon monoxide

| Conditions | Yield |
|---|---|
| With O3Ti(2-)*Ni(2+); ammonia at 499.9℃; Product distribution; other catalysts; |


| Conditions | Yield |
|---|---|
| With ammonium persulfate; ammonium formate In N,N-dimethyl-formamide at 30 - 40℃; | |
| With bromine at 349.9℃; Equilibrium constant; different reagent and reactant concentrations and reaction temperatures; |
-
-
353-59-3
halon-1211

-
A
-
1511-62-2
bromodifluoromethane

-
B
-
75-10-5
Difluoromethane

-
C
-
373-52-4
bromofluoromethane

-
D
-
75-09-2
dichloromethane

-
E
-
75-45-6
Chlorodifluoromethane

-
F
-
74-95-3
1,2-dibromomethane

| Conditions | Yield |
|---|---|
| With hydrogen In gas at 400 - 900℃; under 760 Torr; Rate constant; Product distribution; Mechanism; other reagents; Ea; |

-
-
75-38-7
Vinylidene fluoride

-
-
152239-89-9
C3HClF6O

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
354-34-7
trifluoroacetyl fluoride

| Conditions | Yield |
|---|---|
| In trichlorofluoromethane for 24h; -141 degC to 25 degC; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
116-14-3
polytetrafluoroethylene

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
354-25-6
2-chloro-1,1,2,2-tetrafluoroethane

-
C
-
2837-89-0
1,1,1,2-tetrafluoro-2-chloroethane

-
D
-
115-25-3
Octafluorocyclobutane

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 660℃; under 760 Torr; Mechanism; Ar-diluted mixtures, flow conditions; other temperatures; |

-
-
79-38-9
Chlorotrifluoroethylene

-
-
152239-93-5
C3HCl2F5O

-
A
-
75-45-6
Chlorodifluoromethane

-
B
-
354-27-8
chlorodifluoroacetyl fluoride

| Conditions | Yield |
|---|---|
| In trichlorofluoromethane for 18h; -136 degC to 20 degC; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
75-09-2
dichloromethane

-
A
-
75-10-5
Difluoromethane

-
B
-
593-70-4
R32

-
C
-
75-45-6
Chlorodifluoromethane

-
D
-
75-43-4
Dichlorofluoromethane

-
E
-
75-71-8
Dichlorodifluoromethane

| Conditions | Yield |
|---|---|
| With osmium pentafluoride oxide Product distribution; other transition-metal oxide fluorides; |

-
-
75-71-8
Dichlorodifluoromethane

-
-
812-15-7
1,1,1,2,2-pentamethyldisilane

-
A
-
75-77-4
chloro-trimethyl-silane

-
B
-
993-07-7
trimethylsilan

-
C
-
75-45-6
Chlorodifluoromethane

-
D
-
1560-28-7
pentamethylchlorodisilane

-
E
-
75-78-5
dimethylsilicon dichloride

| Conditions | Yield |
|---|---|
| at 436.9 - 492.9℃; under 1.5 - 3 Torr; Product distribution; Thermodynamic data; other temp.; |


-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| With potassium hydroxide In isopropyl alcohol at 20 - 65℃; for 1.1h; | 100% |
-
-
75-45-6
Chlorodifluoromethane

-
-
944334-01-4
4-bromo-2-vinyloxy-1-methoxybenzene

-
-
944333-99-7
4-bromo-2-cyclopropoxy-1-methoxybenzene

| Conditions | Yield |
|---|---|
| With dimethyl zinc(II) In toluene at -40 - 20℃; for 28.25h; | 100% |
-
-
75-45-6
Chlorodifluoromethane

-
-
140675-43-0
2-Fluoro-6-hydroxybenzonitrile

-
-
221202-14-8
2-(difluoromethoxy)-6-fluorobenzonitrile

| Conditions | Yield |
|---|---|
| With sodium hydroxide In diethylene glycol dimethyl ether; water at 20 - 65℃; | 99% |
| Stage #1: 2-Fluoro-6-hydroxybenzonitrile With sodium hydroxide In 1,2-dimethoxyethane; water at 65℃; Stage #2: Chlorodifluoromethane In 1,2-dimethoxyethane; water at 20 - 65℃; | |
| With sodium hydroxide In 1,4-dioxane; water at 65℃; |
-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran 1.) -78 deg C, 30 min, 2.) -78 deg C, 1 h; | 98% |

| Conditions | Yield |
|---|---|
| With tetrakis(acetonitrile)copper(I)tetrafluoroborate; 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 120℃; for 5h; Mechanism; Sealed tube; Inert atmosphere; | 98% |
-
-
75-45-6
Chlorodifluoromethane

-
-
66225-40-9
N-ethyl-N-methylbutan-1-amine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium nitrate In tetrahydrofuran; water at 20℃; | 97% |
-
-
75-45-6
Chlorodifluoromethane

-
-
144432-79-1
4-biphenylboronic acid 1,2-ethanediol ester

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; hydroquinone In 1,4-dioxane at 110℃; for 48h; Schlenk technique; Inert atmosphere; | 97% |
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; hydroquinone; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 110℃; for 48h; Inert atmosphere; Sealed tube; | 95% |

-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| Stage #1: 5-methyl-2-phenyl-1,2,4-triazolidin-3-one With chlorine In water; 1,2-dichloro-ethane at 20 - 80℃; for 5.36h; Molecular sieve; Stage #2: With potassium carbonate In 5,5-dimethyl-1,3-cyclohexadiene; Triethylene glycol dimethyl ether at 140℃; for 4h; Stage #3: Chlorodifluoromethane In 5,5-dimethyl-1,3-cyclohexadiene; Triethylene glycol dimethyl ether at 185℃; for 0.5h; | 96.1% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 1,4-dioxane; water at 45℃; for 3h; | 96% |
-
-
67249-20-1
1-decylpiperidine

-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 3.5h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: tributyl-amine With sodium hydroxide In tetrahydrofuran; water at 20℃; for 0.0333333h; Stage #2: Chlorodifluoromethane In tetrahydrofuran; water | 96% |
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 3h; | 92% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; hydroquinone In 1,4-dioxane at 110℃; for 48h; Schlenk technique; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With high nickel alloy at 420 - 955℃; for 6.94444E-05h; Temperature; | 95.2% |
| In gas Product distribution; Irradiation; dependence on pressure and light intensity; | |
| Pyrolysis; |
-
-
37622-90-5
4-ethoxycarbonylpyrazole

-
-
75-45-6
Chlorodifluoromethane

-
-
129819-40-5
ethyl 1-(difluoromethyl)-1H-pyrazole-4-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 2h; | 95% |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 130 - 140℃; for 3h; | 41% |
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 130 - 140℃; for 3h; | 41% |
-
-
75-45-6
Chlorodifluoromethane

-
-
123-08-0
4-hydroxy-benzaldehyde

-
-
73960-07-3
4-difluoromethoxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxy-benzaldehyde With sodium hydroxide; 18-crown-6 ether In water; isopropyl alcohol for 0.5h; Stage #2: Chlorodifluoromethane In water; isopropyl alcohol at 65℃; for 5 - 6h; | 95% |
| With sodium hydroxide; sodium dithionite In 1,4-dioxane; water at 65 - 70℃; for 5h; | 76% |
| With sodium hydroxide; Tris(3,6-dioxaheptyl)amine In toluene at 105℃; for 1.17h; | 24% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In N,N-dimethyl-formamide at 60 - 70℃; | 95% |

-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 2h; | 95% |
-
-
120-03-6
2-(p-tolyl)-1H-benzimidazole

-
-
75-45-6
Chlorodifluoromethane

-
-
1309978-33-3
1-(difluoromethyl)-2-(4-methylphenyl)-1H-benzimidazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; | 95% |

| Conditions | Yield |
|---|---|
| With tetrakis(acetonitrile)copper(I)tetrafluoroborate; 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 120℃; for 5h; Mechanism; Sealed tube; Inert atmosphere; | 95% |
-
-
75-45-6
Chlorodifluoromethane

-
-
13922-41-3
1-Naphthylboronic acid

-
-
53731-26-3
1-(difluoromethyl)naphthalene

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; hydroquinone; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 110℃; for 48h; Inert atmosphere; Sealed tube; | 95% |
-
-
75-45-6
Chlorodifluoromethane

-
-
60134-93-2
3-(tert-butyldimethylsilyloxy)oct-1-yne

-
-
183996-60-3
tert-Butyl-[1-(3,3-difluoro-prop-1-ynyl)-hexyloxy]-dimethyl-silane

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran 1.) -78 deg C, 30 min, 2.) -78 deg C, 1 h; | 94% |
-
-
866130-43-0
4-(5-hydroxy-3-trifluoromethyl-pyrazol-1-yl)-benzoic acid ethyl ester

-
-
75-45-6
Chlorodifluoromethane

-
-
866130-44-1
4-(5-difluoromethoxy-3-trifluoromethyl-pyrazol-1-yl)-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at -78 - 100℃; for 12h; | 94% |
-
-
75-45-6
Chlorodifluoromethane

-
-
66-99-9
β-naphthaldehyde

-
-
131581-40-3
1,1-difluoro-2-(2-naphthyl)ethene

| Conditions | Yield |
|---|---|
| With tetrabutyl-ammonium chloride; triphenylphosphine; methyloxirane In N,N-dimethyl-formamide at 110℃; for 6h; Wittig Olefination; Sealed tube; Molecular sieve; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With tetrakis(acetonitrile)copper(I)tetrafluoroborate; 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 120℃; for 5h; Mechanism; Sealed tube; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; hydroquinone In 1,4-dioxane at 110℃; for 48h; Schlenk technique; Inert atmosphere; | 94% |
-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| Stage #1: Chlorodifluoromethane; tetrabutylammonium 7-acetylspiro[1,3-benzodioxole-2,4'-tetrahydrothiopyran]-4-olate In N,N-dimethyl-formamide at 50 - 55℃; Large scale; Stage #2: With water; sodium hydroxide In N,N-dimethyl-formamide at 45 - 55℃; Large scale; | 94% |

| Conditions | Yield |
|---|---|
| With dmap; 4,4'-diamino-2,2'-bipyridyl; magnesium chloride; nickel dichloride; zinc In N,N-dimethyl acetamide at 60℃; for 12h; Molecular sieve; | 94% |
-
-
75-45-6
Chlorodifluoromethane

| Conditions | Yield |
|---|---|
| With selenium; water; potassium carbonate In N,N-dimethyl-formamide at 120℃; for 10h; Schlenk technique; Inert atmosphere; regioselective reaction; | 94% |
Difluorochloromethane Chemical Properties
Molecular Formula: CHClF2
Molecular Weight: 86.47
EINECS: 200-871-9
Index of Refraction: 1.278
Density: 1.277 g/cm3
Melting point: -146 °C
Boiling Point: 155 °C
Water solubility: Slightly soluble
Appearance: Colourless gas
Structure of Difluorochloromethane (CAS NO.75-45-6):
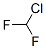
IUPAC Name: Chloro(difluoro)methane
Product Category of Difluorochloromethane (CAS NO.75-45-6): refrigerants;Organics;Refrigerant
Difluorochloromethane Uses
Difluorochloromethane (CAS NO.75-45-6) is mainly used as mainlyPropellant, fumigant, insecticide. It was once commonly used as a propellant and in air conditioning applications.
Difluorochloromethane Production
Difluorochloromethane (CAS NO.75-45-6) can be prepared from Chloroform : HCCl3 + 2HF → HCF2Cl + 2HCl .
Difluorochloromethane Toxicity Data With Reference
| 1. | mmo-sat 33 pph/24H-C | TOLED5 Toxicology Letters. 2 (1978),1. | ||
| 2. | mma-sat 33 pph/24H-C | TOLED5 Toxicology Letters. 2 (1978),1. | ||
| 3. | ihl-rat LC50:35 pph/15M | HUTODJ Human Toxicology. 1 (1982),239. | ||
| 4. | ihl-mus LC50:28 pph/20M | TXAPA9 Toxicology and Applied Pharmacology. 59 (1981),64. | ||
| 5. | ihl-dog LCLo:70 pph | TXAPA9 Toxicology and Applied Pharmacology. 2 (1960),363. |
Difluorochloromethane Consensus Reports
It is reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Difluorochloromethane Safety Profile
Hazard Codes:  F
F
Risk Statements: 40-59
R40:Limited evidence of a carcinogenic effect.
R59:Dangerous to the ozone layer.
Safety Statements: 23-59
S23:Do not breathe vapour.
S59:Refer to manufacturer / supplier for information on recovery / recycling.
Mildly toxic by inhalation. Experimental reproductive effects. Mutation data reported. An asphyxiant in high concentrations. At elevated pressures, 50% mixtures with air are combustible although ignition is difficult. When heated to decomposition it emits toxic fumes of F− and Cl−.
Difluorochloromethane Standards and Recommendations
OSHA PEL: TWA 1000 ppm
ACGIH TLV: TWA 1000 ppm; Not Classifiable as a Human Carcinogen
DFG MAK: 500 ppm (1800 mg/m3)
DOT Classification: 2.2; Label: Nonflammable Gas
Difluorochloromethane Specification
Difluorochloromethane , its cas register number is 75-45-6. It also can be called Chlorodifluoromethane ; Fluorocarbon 22 ; Freon R-22 ; and Freon 22 . Difluorochloromethane (CAS NO.75-45-6) is shipped as a liquefied gas under its own vapor pressure, and is noncombustible. Difluorochloromethane can asphyxiate by the displacement of air. Contact with the liquid can cause frostbite.
Related Products
- Difluorochloromethane
- 7545-71-3
- 75462-59-8
- 75462-61-2
- 7546-27-2
- 75464-92-5
- 75-46-7
- 75467-36-6
- 754-67-6
- 75472-91-2
- 75473-09-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View