-
Name
Dihydroterpineol
- EINECS 207-871-8
- CAS No. 498-81-7
- Article Data29
- CAS DataBase
- Density 0.9 g/cm3
- Solubility
- Melting Point
- Formula C10H20O
- Boiling Point 212.223 °C at 760 mmHg
- Molecular Weight 156.268
- Flash Point 89.708 °C
- Transport Information
- Appearance Clear liquid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms p-Menthan-8-ol(6CI,7CI,8CI);8-p-Menthanol;Dihydro-a-terpineol;Cyclohexanemethanol, a,a,4-trimethyl-;2-(4-Methylcyclohexyl)-2-propanol;alpha,alpha,4-Trimethylcyclohexanemethanol;p-Menthan-8-ol;
- PSA 20.23000
- LogP 2.58360
Synthetic route

| Conditions | Yield |
|---|---|
| With lithium In ethylenediamine for 0.5h; | A 95% B 5% |
| With lithium aluminium tetrahydride; aluminium trichloride In diethyl ether for 8h; Ambient temperature; | A 23% B 70% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol for 8h; | 93.5% |
| With 5%-palladium/activated carbon; hydrogen In methanol for 12h; | 93.5% |
| With hydrogen; nickel Hydrogenation; |

-
A
-
498-81-7
p-menthan-8-ol

-
B
-
3901-93-7, 3901-95-9, 21129-27-1
1-methyl-4-(1-methylethyl)cyclohexanol

-
C
-
470-65-5
p-menthane-4-ol

| Conditions | Yield |
|---|---|
| With deuterioporphyrin dimethyl ester ferric chloride In dichloromethane at 130℃; for 18h; Catalytic behavior; Reagent/catalyst; Temperature; | A 34.44% B 5.71% C 49.16% |
| With C32H32CoN4O4 at 130℃; under 760.051 Torr; for 17h; Reagent/catalyst; Overall yield = 10.2 %; chemoselective reaction; |

-
-
917-64-6
methyl magnesium iodide

-
-
41692-50-6
trans-4-methyl-1-(ethoxycarbonyl)cyclohexane

-
-
498-81-7
p-menthan-8-ol

| Conditions | Yield |
|---|---|
| With copper substances of uncertain configuration; | |
| With nickel substances of uncertain configuration; |

| Conditions | Yield |
|---|---|
| With palladium-catalysts at 100℃; substances of uncertain configuration; |

| Conditions | Yield |
|---|---|
| With benzoic acid In water at 100℃; Product distribution; sealed tube; | 37 % Chromat. |
-
-
7133-31-5
4-methylcyclohexanecarboxylic acid ethyl ester

-
-
74-88-4
methyl iodide

-
-
498-81-7
p-menthan-8-ol

| Conditions | Yield |
|---|---|
| With magnesium 1.)Et2O; Yield given. Multistep reaction; |

-
-
498-81-7
p-menthan-8-ol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol; platinum Hydrogenation.Behandlung des Reaktionsprodukts mit aethanol. Alkalilauge; substances of uncertain configuration; |

| Conditions | Yield |
|---|---|
| With diethyl ether |

-
-
638-38-0, 993-02-2, 2180-18-9, 15411-95-7, 22981-23-3, 27004-39-3
manganese(II) acetate

-
-
301-04-2
lead acetate

-
A
-
498-81-7
p-menthan-8-ol

-
B
-
4331-54-8
4-methylcyclohexanecarboxylic acid

-
C
-
62067-45-2
4-isopropyl cyclohexane carboxylic acid

| Conditions | Yield |
|---|---|
| at 30 - 50℃; auch bei 100grad; substance of uncertain configuration; |


| Conditions | Yield |
|---|---|
| With nickel Hydrogenation; trans-dihydro-α-terpineol; |

| Conditions | Yield |
|---|---|
| at 150℃; Kinetics; Hydrogenation; dl-α-terpineol; | |
| Hydrogenation; dl-α-terpineol; |

| Conditions | Yield |
|---|---|
| Hydrogenation; dl-α-terpineol; |

| Conditions | Yield |
|---|---|
| at 200 - 240℃; under 58840.6 - 73550.8 Torr; aus Sho-gyu-Oel isoliertes Praeparat.Hydrogenation; |


| Conditions | Yield |
|---|---|
| at 100℃; |



-
-
22273-97-8
methyl-4 cyclohexenyl-1 methyle cetone

-
-
498-81-7
p-menthan-8-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water; palladium / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water; palladium / Hydrogenation View Scheme |

| Conditions | Yield |
|---|---|
| With Aspergillus niger (P.T.C.C.5011); Sabouraud Dextrose Agar |
-
-
1678-82-6
trans-1,4-menthane

-
A
-
498-81-7
p-menthan-8-ol


-
C
-
3901-95-9
cis p-menthanol

-
D
-
470-65-5
p-menthane-4-ol

| Conditions | Yield |
|---|---|
| With monooxygenase P450-BM3; nicotinamide adenine dinucleotide phosphate for 3h; Catalytic behavior; Enzymatic reaction; stereoselective reaction; |


| Conditions | Yield |
|---|---|
| With monooxygenase P450-BM3 (A328F) mutant; nicotinamide adenine dinucleotide phosphate for 3h; Catalytic behavior; Enzymatic reaction; stereoselective reaction; |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; zinc dibromide | 80% |
| With hydrogen bromide at 50℃; im Druckrohr; |
-
-
10026-04-7, 53609-55-5
tetrachlorosilane

-
-
498-81-7
p-menthan-8-ol

-
-
18724-26-0
Dichlor-bis-(p-menthan-8-yloxy)-silan

| Conditions | Yield |
|---|---|
| With pyridine In benzene | 80% |
| With pyridine In benzene | 80% |
| With pyridine In benzene | 80% |

| Conditions | Yield |
|---|---|
| With sulfuric acid Ambient temperature; | 48% |

| Conditions | Yield |
|---|---|
| With sulfuric acid Ambient temperature; | 37% |

| Conditions | Yield |
|---|---|
| With oxalic acid | |
| With sulfuric acid; acetic acid |

| Conditions | Yield |
|---|---|
| With phosphorus pentaoxide inactive p-menthene-(3); | |
| With oxalic acid inactive p-menthene-(3); | |
| With potassium pyrosulfate at 200℃; im Kupferautoklaven; inactive p-menthene-(3); | |
| With magnesium aluminium-silicate at 300℃; | |
| With magnesium aluminium-silicate at 300℃; |

| Conditions | Yield |
|---|---|
| With potassium hydrogensulfate |

| Conditions | Yield |
|---|---|
| With water; magnesium chloride at 230 - 240℃; |
-
-
498-81-7
p-menthan-8-ol

-
-
91138-81-7
8-chloro-p-menthane

| Conditions | Yield |
|---|---|
| With hydrogenchloride; diethyl ether at 0℃; |
-
-
498-81-7
p-menthan-8-ol

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; benzene |

| Conditions | Yield |
|---|---|
| With phosphoric acid |

| Conditions | Yield |
|---|---|
| es wurden zwei Verbindungen vom Schmelzpunkt 89-91grad und 116-117grad erhalten; |
-
-
59965-20-7, 72746-96-4, 79434-89-2, 3383-83-3
1-bromo-3,7-dimethyloctane

-
-
498-81-7
p-menthan-8-ol

-
-
57706-83-9
1-[1-(3,7-Dimethyl-octyloxy)-1-methyl-ethyl]-4-methyl-cyclohexane

| Conditions | Yield |
|---|---|
| (i) Na, Et2O, (ii) /BRN= 1719643/; Multistep reaction; |
-
-
498-81-7
p-menthan-8-ol

-
-
24302-23-6, 83206-95-5, 18479-65-7
p-menth-3-en-8-ol

| Conditions | Yield |
|---|---|
| (i) (dehydratation), (ii) (UV-irradiation), O2, rose bengale, (iii) Ph3P; Multistep reaction; |

| Conditions | Yield |
|---|---|
| (i) (dehydratation), (ii) (UV-irradiation), O2, rose bengale, (iii) Ph3P; Multistep reaction; |

| Conditions | Yield |
|---|---|
| at 300℃; substances of uncertain configuration; | |
| at 300℃; substances of uncertain configuration; |
Dihydroterpineol Consensus Reports
Dihydroterpineol Specification
The Dihydroterpineol, with the CAS registry number 498-81-7, is also known as alpha,alpha,4-Trimethylcyclohexanemethanol. It belongs to the product category of Pharmaceutical Intermediates. Its EINECS number is 207-871-8. This chemical's molecular formula is C10H20O and molecular weight is 156.27. What's more, its systematic name is 2-(4-Methylcyclohexyl)-2-propanol. Its classification code is Skin / Eye Irritant.
Physical properties of Dihydroterpineol are: (1)ACD/LogP: 3.092; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.09; (4)ACD/LogD (pH 7.4): 3.09; (5)ACD/BCF (pH 5.5): 131.76; (6)ACD/BCF (pH 7.4): 131.76; (7)ACD/KOC (pH 5.5): 1145.38; (8)ACD/KOC (pH 7.4): 1145.38; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.459; (14)Molar Refractivity: 47.476 cm3; (15)Molar Volume: 173.691 cm3; (16)Polarizability: 18.821×10-24cm3; (17)Surface Tension: 31.10 dyne/cm; (18)Density: 0.9 g/cm3; (19)Flash Point: 89.708 °C; (20)Enthalpy of Vaporization: 52.168 kJ/mol; (21)Boiling Point: 212.223 °C at 760 mmHg; (22)Vapour Pressure: 0.039 mmHg at 25°C.
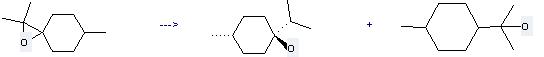
Preparation: this chemical can be prepared by p-4(8)-Menthene oxide at the ambient temperature. This reaction will need reagents LiAlH4, AlCl3 and solvent diethyl ether with the reaction time of 8 hours. The yield is about 70%.
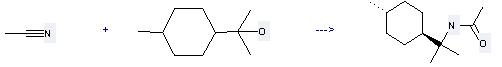
Uses of Dihydroterpineol: it can be used to produce N-p-menth-8-ylacetamide at the temperature of 20 °C. It will need reagent H2SO4 with the reaction time of 2 days. The yield is about 95%.
You can still convert the following datas into molecular structure:
(1)SMILES: OC(C)(C)C1CCC(C)CC1
(2)Std. InChI: InChI=1S/C10H20O/c1-8-4-6-9(7-5-8)10(2,3)11/h8-9,11H,4-7H2,1-3H3
(3)Std. InChIKey: UODXCYZDMHPIJE-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 12, Pg. 529, 1974. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 12, Pg. 529, 1974. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View