-
Name
Dimethyl acetylmethylphosphonate
- EINECS 224-110-5
- CAS No. 4202-14-6
- Article Data34
- CAS DataBase
- Density 1.145 g/cm3
- Solubility
- Melting Point 76-79 °C at 3 mm Hg
- Formula C5H11O4P
- Boiling Point 227 °C at 760 mmHg
- Molecular Weight 166.114
- Flash Point 105.3 °C
- Transport Information
- Appearance Clear, colorless liquid.
- Safety 23-24/25
- Risk Codes
-
Molecular Structure
-
Hazard Symbols

- Synonyms Phosphonicacid, (2-oxopropyl)-, dimethyl ester (9CI);Phosphonic acid, acetonyl-,dimethyl ester (6CI,7CI,8CI);(2-Oxopropyl)phosphonic acid dimethyl ester;(Dimethylphosphono)acetone;Dimethyl (2-oxopropyl)phosphonate;Dimethylacetonylphosphonate;Dimethyl b-keto-propylphosphonate;
- PSA 62.41000
- LogP 1.06130
Synthetic route
-
-
78-95-5
chloroacetone

-
-
121-45-9
phosphorous acid trimethyl ester

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| In toluene at 135℃; under 3750.38 - 15001.5 Torr; for 0.333333h; Arbuzov Reaction; Large scale; | 88% |
| Stage #1: chloroacetone With potassium iodide In acetone; acetonitrile at 20℃; for 2h; Stage #2: phosphorous acid trimethyl ester In acetone; acetonitrile at 50℃; for 24h; | 82% |
| Stage #1: chloroacetone With potassium iodide In acetone; acetonitrile at 20℃; for 2h; Irradiation; Stage #2: phosphorous acid trimethyl ester In acetone; acetonitrile at 50℃; for 24h; | 77% |
-
-
103517-85-7, 103538-61-0
[2-(Methoxycarbonyl-hydrazono)-propyl]-phosphonic acid dimethyl ester

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetone for 3h; Ambient temperature; | 82% |
-
-
3019-04-3
iodoacetone

-
-
121-45-9
phosphorous acid trimethyl ester

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| In toluene for 2h; Heating; | 74% |
| In benzene | |
| In acetone; acetonitrile at 20℃; for 12h; Michaelis-Arbuzov reaction; | 101.3 g |
| In acetone; acetonitrile at 20 - 50℃; Inert atmosphere; | 41 g |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water; N,N-dimethyl-formamide at 85℃; for 24h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 72% |

| Conditions | Yield |
|---|---|
| With potassium iodide; phosphorous acid trimethyl ester In acetone; acetonitrile | 71% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In water at 85℃; Inert atmosphere; Schlenk technique; chemoselective reaction; | 60% |
-
-
79-20-9
acetic acid methyl ester

-
-
756-79-6
dimethyl methane phosphonate

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl methane phosphonate With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: acetic acid methyl ester In tetrahydrofuran; hexane at -78 - 0℃; Further stages.; | 56% |
| Stage #1: dimethyl methane phosphonate With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: acetic acid methyl ester In tetrahydrofuran; hexane at -78 - 0℃; | 55% |
| Stage #1: dimethyl methane phosphonate With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 3h; Stage #2: acetic acid methyl ester In tetrahydrofuran; hexane at -78 - 0℃; | |
| With lithium diisopropyl amide In tetrahydrofuran at -5 - 0℃; Inert atmosphere; |
-
-
141-78-6
ethyl acetate

-
-
756-79-6
dimethyl methane phosphonate

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl methane phosphonate With n-butyllithium In hexane; toluene at -80℃; for 0.166667h; Inert atmosphere; Stage #2: ethyl acetate In hexane; toluene at -80℃; for 1h; Inert atmosphere; | 50% |
| With n-butyllithium 1.) THF, -78 deg C, 15 min, 2.) THF, -78 deg C, 30 min; Multistep reaction; |
-
-
876-27-7
4-chlorophenyl acetate

-
-
54731-75-8
dimethyl (2-hydroxypropyl)phosphonate

-
A
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With (hydroxy)tetraphenylcyclopentadienyl-based ruthenium; Candida antarctica lipase B (Novozym-435) In toluene at 70℃; for 24h; | A 16% B 62 % Spectr. |

-
-
78-95-5
chloroacetone

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
4185-82-4
phosphoric acid isopropenyl ester dimethyl ester

-
B
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
75-36-5
acetyl chloride

-
-
756-79-6
dimethyl methane phosphonate

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| (i) nBuLi, hexane, THF, (ii) Cu2I2, (iii) /BRN= 605303/, Et2O; Multistep reaction; |
-
-
598-31-2
1-bromoacetone

-
-
121-45-9
phosphorous acid trimethyl ester

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| In methanol Heating; | |
| at 120℃; for 1.5h; |
-
-
67-56-1
methanol

-
-
90965-06-3
dimethyl 1-(1-diazo-2-oxopropyl)phosphonate

-
A
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
B
-
26530-60-9
trimethyl 2-phosphonopropionate

| Conditions | Yield |
|---|---|
| at 0℃; Irradiation; | A 12.3 % Chromat. B 87.5 % Chromat. |

-
-
90965-06-3
dimethyl 1-(1-diazo-2-oxopropyl)phosphonate

-
A
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
B
-
26530-60-9
trimethyl 2-phosphonopropionate

| Conditions | Yield |
|---|---|
| In methanol at 0℃; Irradiation; | A 12.3 % Chromat. B 87.5 % Chromat. |
-
-
56504-60-0
1,2-propenephosphonic acid methyl ester

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With water; oxygen; p-benzoquinone; lithium chloride; palladium dichloride at 65℃; for 26h; Yield given; |
-
-
58393-50-3
methyl 2-dimethoxyphosphoryl-3-oxobutyrate

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With water for 2.5h; Heating; Yield given; |
-
-
78-95-5
chloroacetone

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
598-31-2
1-bromoacetone

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: KOtBu 2: LiCl, p-benzoquinone, O2, H2O / PdCl2 / 26 h / 65 °C View Scheme |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
90965-06-3
dimethyl 1-(1-diazo-2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With sodium hydride In tetrahydrofuran; mineral oil; benzene at 20℃; for 1h; Inert atmosphere; Cooling with ice; Stage #2: With 4-toluenesulfonyl azide In tetrahydrofuran; mineral oil; benzene at 20℃; Inert atmosphere; | 100% |
| Stage #1: dimethyl (2-oxopropyl)phosphonate With sodium hydride In tetrahydrofuran; mineral oil; benzene at 20℃; for 1h; Inert atmosphere; Cooling with ice; Stage #2: With 4-toluenesulfonyl azide In tetrahydrofuran; mineral oil; benzene at 20℃; Inert atmosphere; | 99% |
| With 4-toluenesulfonyl azide In tetrahydrofuran; benzene at 0 - 20℃; for 2h; Substitution; | 98% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
55420-70-7
(E)-4-(3,4-dichlorophenyl)but-3-en-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 5℃; for 0.25h; | 100% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With potassium tert-butylate In tert-butyl alcohol at 20℃; for 0.5h; Stage #2: With acetyl hypofluorite In tert-butyl alcohol at 20℃; | 100% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With RuCl2((R)-binap)(dmf)n; hydrogen In methanol at 25℃; under 3040 Torr; for 72h; | 99% |
| Multi-step reaction with 3 steps 1.1: sodium hydride / tetrahydrofuran; mineral oil / 0.5 h / Inert atmosphere 1.2: 16 h / Inert atmosphere 2.1: BF4(1-)*C50H59IrO3P2(1+); hydrogen / dichloromethane / 24 h / 25 °C / 3040.2 Torr 3.1: sodium carbonate / methanol / 16 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium hydride / tetrahydrofuran; mineral oil / 0.5 h / Inert atmosphere 1.2: 16 h / Inert atmosphere 2.1: BF4(1-)*C44H63IrO3P2(2+); hydrogen / dichloromethane / 24 h / 25 °C / 3040.2 Torr 3.1: sodium carbonate / methanol / 16 h View Scheme |
-
-
32133-82-7
Martins sulfurane

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
1433220-51-9
dimethyl 2-(diphenylsulfuranylidene)-2-oxopropylphosphonate

| Conditions | Yield |
|---|---|
| In diethyl ether at 25℃; for 0.5h; Schlenk technique; Inert atmosphere; | 99% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
555-16-8
4-nitrobenzaldehdye

-
-
937-31-5
(4-Nitrophenyl)acetylene

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 20℃; for 18h; Seyferth-Gilbert Homologation; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With 4-toluenesulfonyl azide; potassium carbonate In acetonitrile at 25℃; for 2h; Inert atmosphere; Stage #2: C32H42F2N2O6Si2 In methanol; acetonitrile at 25℃; for 12h; Inert atmosphere; | 98.2% |
| Stage #1: dimethyl (2-oxopropyl)phosphonate With 4-toluenesulfonyl azide; potassium carbonate In acetonitrile at 25℃; for 2h; Inert atmosphere; Stage #2: C32H42F2N2O6Si2 In methanol at 25℃; for 12h; Inert atmosphere; | 98.2% |
| Stage #1: dimethyl (2-oxopropyl)phosphonate With 4-toluenesulfonyl azide; potassium carbonate In acetonitrile at 25℃; for 2h; Inert atmosphere; Stage #2: C32H42F2N2O6Si2 In methanol; acetonitrile at 25℃; for 12h; | 98.2% |

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With sodium hydride In tetrahydrofuran; benzene at 0℃; for 1h; Stage #2: 4-toluenesulfonyl azide In tetrahydrofuran; benzene at 20℃; for 2h; | 98% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With n-butyllithium In tetrahydrofuran; hexane at -70℃; for 0.25h; Inert atmosphere; Stage #2: C28H30N2O5 In tetrahydrofuran; hexane at -70℃; for 2h; Inert atmosphere; | 98% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
106889-84-3
2-N,N-Dibenzylaminoacetaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With lithium bromide In tetrahydrofuran at 20℃; for 0.0833333h; Horner-Wadsworth-Emmons Olefination; Inert atmosphere; Stage #2: With triethylamine In tetrahydrofuran for 0.166667h; Inert atmosphere; Stage #3: 2-N,N-Dibenzylaminoacetaldehyde In tetrahydrofuran for 0.75h; Inert atmosphere; | 98% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
15186-48-8
2,3-isopropylidene-glyceraldehyde

-
-
64482-41-3
(3E)-4-[(4S)-2,2-dimethyl(1,3-dioxolan-4-yl)]but-3-en-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran; water at 0℃; for 1h; | 97% |
| With sodium hydride 1.) THF 1.5h at room temp.; 2.) 2 h from 0 degC to 5 degC; Yield given. Multistep reaction; | |
| With potassium carbonate at 0℃; for 3h; Yield given; | |
| With potassium carbonate |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
226065-51-6
(S)-2-[(R)-3-(tert-Butyl-dimethyl-silanyloxy)-1-methyl-propyl]-2,5-dimethyl-hex-4-enal

-
-
226065-52-7
(E)-(S)-5-[(R)-3-(tert-Butyl-dimethyl-silanyloxy)-1-methyl-propyl]-5,8-dimethyl-nona-3,7-dien-2-one

| Conditions | Yield |
|---|---|
| With n-butyllithium In toluene Heating; | 97% |
-
-
887113-19-1
4-methyl-N-[(2S)-3-methyl-1-oxobutan-2-yl]benzenesulfonamide

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With N-ethyl-N,N-diisopropylamine; lithium chloride In acetonitrile at 0℃; for 0.333333h; Stage #2: 4-methyl-N-[(2S)-3-methyl-1-oxobutan-2-yl]benzenesulfonamide In acetonitrile at 20℃; for 2h; | 97% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
88631-43-0
(2-Cyano-2-methyl-2-trimethylsilanyloxy-ethyl)-phosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With potassium cyanide; 18-crown-6 ether In dichloromethane for 2h; | 96% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
139338-88-8
4-(Tetrahydro-pyran-2-yloxy)-non-5-ynal

-
-
139338-89-9
(E)-7-(Tetrahydro-pyran-2-yloxy)-dodec-3-en-8-yn-2-one

| Conditions | Yield |
|---|---|
| With sodium hydride | 96% |
| With sodium hydride | 92% |
-
-
862509-80-6
(2S,3R,4R)-2,3-bis-(p-methoxybenzyloxy)-4-[5-(R)-(p-methoxybenzyloxy)-2,2,6-(6R)-trimethyl-1,3-dioxan-4-(R)-yl]-pentanal

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
392692-56-7
(3E,5R,6R,7R)-5,6-bis-(p-methoxybenzyloxy)-7-[5-(R)-(p-methoxybenzyloxy)-2,2,6-(6R)-trimethyl-1,3-dioxan-4-(R)-yl]-oct-3-en-2-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With barium dihydroxide In tetrahydrofuran at 20℃; for 0.5h; Stage #2: (2S,3R,4R)-2,3-bis-(p-methoxybenzyloxy)-4-[5-(R)-(p-methoxybenzyloxy)-2,2,6-(6R)-trimethyl-1,3-dioxan-4-(R)-yl]-pentanal In tetrahydrofuran; water at 20℃; for 4h; Horner-Wadsworth-Emmons chain extension; | 96% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With C48H44ClO4P2Ru(1+)*Cl(1-); hydrogen In methanol at 50℃; under 7500.75 Torr; for 19h; Inert atmosphere; enantioselective reaction; | 95.3% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
146000-77-3
ethyl (E)-3-((E)-carbamoyldiazenyl)but-2-enoate

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 2h; Ambient temperature; | 95% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
108-94-1
cyclohexanone

-
-
874-68-0
1-cyclohexylidene-2-propanone

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water at 20℃; for 28h; Wittig-Horner reaction; | 95% |
-
-
29943-42-8
Tetrahydro-4H-pyran-4-one

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
912441-79-3
1-(dihydro-2H-pyran-4(3H)-ylidene)propan-2-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water at 0 - 20℃; for 5h; Horner-Wadsworth-Emmons Olefination; Inert atmosphere; | 95% |
| With potassium hydroxide In ethanol; water at 20℃; for 5h; | 82% |
| With potassium hydroxide In ethanol at 0 - 20℃; | |
| With potassium hydroxide In ethanol; water at 0 - 20℃; | |
| With potassium hydroxide In ethanol; water at 0℃; for 4h; |

| Conditions | Yield |
|---|---|
| With tetramethylguanidinium acetate at 50℃; for 1.5h; Ionic liquid; | 95% |

| Conditions | Yield |
|---|---|
| With C5H14N3(1+)*HO(1-) at 50℃; for 2h; Ionic liquid; | 95% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
122-51-0
orthoformic acid triethyl ester

-
-
73542-47-9
dimethyl 2-ethoxypropenylphosphonate

| Conditions | Yield |
|---|---|
| With iron(III) chloride for 72h; Ambient temperature; | 94% |
| With iron(III) chloride | |
| With iron(III) chloride Substitution; |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
109613-36-7
(R)-3-((tert-butyldimethylsilyl)oxy)-1-iodobutane

-
-
121484-92-2
dimethyl (6R)-(6-[(tert-butyldimethylsilyl)oxy]-2-oxoheptyl)phosphonate

| Conditions | Yield |
|---|---|
| With n-butyllithium; sodium hydride In tetrahydrofuran at 0℃; for 30h; | 94% |
-
-
799852-58-7
4-[(2R,6R)-4-Methylene-6-(2-oxo-ethyl)-tetrahydro-pyran-2-yl]-but-2-ynoic acid methyl ester

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
799852-60-1
4-[(2R,6S)-4-Methylene-6-((E)-4-oxo-pent-2-enyl)-tetrahydro-pyran-2-yl]-but-2-ynoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With barium dihydroxide In tetrahydrofuran for 0.5h; Stage #2: 4-[(2R,6R)-4-Methylene-6-(2-oxo-ethyl)-tetrahydro-pyran-2-yl]-but-2-ynoic acid methyl ester In tetrahydrofuran; water for 1.75h; Horner-Wadsworth-Emmons reaction; | 94% |

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 24h; Reflux; Dean-Stark; | 94% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
364631-73-2
tert-butyl 5-formyl-2,2-dimethyl-1,3-dioxan-5-ylcarbamate

-
-
364631-74-3
5-ethynyl-2,2-dimethyl-[1,3]dioxan-(N-tert-butyloxycarbonyl)-5-ylamine

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With 4-toluenesulfonyl azide; potassium carbonate In toluene; acetonitrile at 20℃; for 2.5h; Stage #2: tert-butyl 5-formyl-2,2-dimethyl-1,3-dioxan-5-ylcarbamate In methanol; toluene; acetonitrile for 1.5h; | 93.4% |
| Stage #1: dimethyl (2-oxopropyl)phosphonate With 4-toluenesulfonyl azide; potassium carbonate In acetonitrile at 20℃; for 2h; Stage #2: tert-butyl 5-formyl-2,2-dimethyl-1,3-dioxan-5-ylcarbamate In methanol; acetonitrile at 20℃; for 8h; | 84% |
-
-
177947-96-5
tert-butyl 3-formylazetidine-1-carboxylate

-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
287193-01-5
tert-butyl 3-ethynylazetidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With sodium azide; caesium carbonate; methanesulfonyl chloride In acetonitrile at 0 - 20℃; for 3h; Stage #2: tert-butyl 3-formylazetidine-1-carboxylate With caesium carbonate In methanol; acetonitrile at 0℃; | 93% |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
132298-32-9
methyl (E)-4,4-dimethyl-6-oxo-2-hexenoate

-
-
72808-49-2
methyl (E,E)-4,4-dimethyl-8-oxo-2,6-nonadienoate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl (2-oxopropyl)phosphonate With sodium hydride In tetrahydrofuran at 0℃; for 1h; Stage #2: methyl (E)-4,4-dimethyl-6-oxo-2-hexenoate In tetrahydrofuran Horner-Wadsworth-Emmons olefination; Heating; Further stages.; | 92% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; Horner-Emmons olefination; | 92% |
Dimethyl acetylmethylphosphonate Chemical Properties
Molecule structure of Phosphonic acid,P-(2-oxopropyl)-, dimethyl ester (CAS NO.4202-14-6):
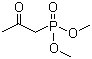
IUPAC Name: 1-Dimethoxyphosphorylpropan-2-one
Molecular Weight: 166.112201 [g/mol]
Molecular Formula: C5H11O4P
Index of Refraction: 1.405
Molar Refractivity: 35.6 cm3
Molar Volume: 145 cm3
Surface Tension: 32.9 dyne/cm
Density: 1.145 g/cm3
Flash Point: 105.3 °C
Enthalpy of Vaporization: 46.36 kJ/mol
Melting Point: 76-79 °C at 3 mm Hg(lit.)
Boiling Point: 227 °C at 760 mmHg
Vapour Pressure: 0.0793 mmHg at 25 °C
XLogP3: 0.9
H-Bond Acceptor: 4
Rotatable Bond Count: 4
Tautomer Count: 3
Exact Mass: 166.039495
MonoIsotopic Mass: 166.039495
Topological Polar Surface Area: 52.6
Heavy Atom Count: 10
Canonical SMILES: CC(=O)CP(=O)(OC)OC
InChI: InChI=1S/C5H11O4P/c1-5(6)4-10(7,8-2)9-3/h4H2,1-3H3
InChIKey: UOWIYNWMROWVDG-UHFFFAOYSA-N
EINECS: 224-110-5
Product Categories of Phosphonic acid,P-(2-oxopropyl)-, dimethyl ester (CAS NO.4202-14-6): Horner-Emmons Reaction; Synthetic Organic Chemistry; Wittig & Horner-Emmons Reaction
Dimethyl acetylmethylphosphonate Safety Profile
Hazard Codes:  Xi
Xi
Safety Statements: 23-24/25
S23:Do not breathe vapour.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
F: 10
Hazard Note: Irritant
Dimethyl acetylmethylphosphonate Specification
Phosphonic acid,P-(2-oxopropyl)-, dimethyl ester (CAS NO.4202-14-6) is also called Dimethyl (2-oxopropyl)phosphonate ; Dimethyl acetylmethylphosphonate ; Dimethyl acetonylphosphonate ; Dimethyl 2-oxopropylphosphonate .
Related Products
- Dimethyl 1,3-adamantanedicarboxylate
- Dimethyl 1,4-cubanedicarboxylate
- Dimethyl 1,4-cyclohexanedicarboxylate
- Dimethyl 2-(2-methoxyphenoxy)malonate
- Dimethyl 2-(2-phenylsulfanylethyl)propanedioate
- Dimethyl 2-(pyrimidin-4-yl)malonate
- Dimethyl 2,2'-azobis(2-methylpropionate)
- Dimethyl 2,2'-oxybisacetate
- Dimethyl 2,2'-thiobisacetate
- Dimethyl 2,3-quinolinedicarboxylate
- 42023-59-6
- 4202-38-4
- 42024-67-9
- 42024-93-1
- 420-25-7
- 4203-18-3
- 4203-49-0
- 4203-50-3
- 42036-65-7
- 420-37-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View