-
Name
Diphenhydramine
- EINECS 200-396-7
- CAS No. 58-73-1
- Article Data39
- CAS DataBase
- Density 1.025 g/cm3
- Solubility
- Melting Point 167-172 °C
- Formula C17H21NO
- Boiling Point 343.734 °C at 760 mmHg
- Molecular Weight 255.36
- Flash Point 101.53 °C
- Transport Information
- Appearance clear light yellow liquid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Ethanamine, 2-(diphenylmethoxy)-N,N-dimethyl-;Ethylamine,2-(diphenylmethoxy)-N,N-dimethyl- (7CI,8CI);2-(Diphenylmethoxy)-N,N-dimethylethylamine;Benzhydramine;Dimedrol base;FAR 90X2;N-[2-(Diphenylmethoxy)ethyl]-N,N-dimethylamine;O-Benzhydryldimethylaminoethanol;Probedryl;a-(2-Dimethylaminoethoxy)diphenylmethane;b-Dimethylaminoethylbenzhydrylether;N-[2-(Diphenylmethoxy)ethyl]-N,N-dimethylamine;
- PSA 12.47000
- LogP 3.35420
Synthetic route
-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
91-01-0
1,1-Diphenylmethanol

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With di(n-butyl)tin oxide; 3-methyl-1-propylimidazolium bromide In neat (no solvent) for 8h; Reagent/catalyst; Reflux; | 98% |
-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
90-99-3
diphenylchloromethane

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 175℃; under 12929 Torr; for 0.266667h; | 93% |
| In acetonitrile at 200℃; for 0.0833333h; Temperature; |
-
-
91-01-0
1,1-Diphenylmethanol

-
-
107-99-3
2-(dimethylamino)ethyl chloride

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With sodium hydride In benzene 1. room temperature, 90 min; 2. 60 deg C, 30 min; | 92% |
-
-
67-56-1
methanol

-
-
17471-10-2
Desmethyldiphenhydramine

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; water In acetonitrile at 20℃; Irradiation; | 88% |
-
-
26926-47-6
2-diphenylmethoxyethanol

-
-
124-40-3
dimethyl amine

-
A
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
B
-
1119277-62-1
2-(dimethylamino)-3,3-diphenylpropan-1-ol

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; bis[2-(diphenylphosphino)phenyl] ether In toluene at 20℃; Inert atmosphere; Reflux; | A 10% B 67% |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 60℃; for 36h; | 35% |
-
-
41858-14-4
2-(Diphenylmethoxy)-N,N-dimethylacetamid

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether |
-
-
32669-06-0
1,1'-[(2-chloroethoxy)methylene]bis-benzene

-
-
124-40-3
dimethyl amine

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine
-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
776-74-9
Bromodiphenylmethane

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With sodium carbonate; benzene at 120 - 125℃; | |
| In acetonitrile at 200℃; for 0.166667h; Temperature; | |
| In toluene for 20h; Williamson Ether Synthesis; Reflux; |
-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
908093-98-1
diazodiphenylmethane

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With benzene |

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| at 190℃; |

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| at 230 - 300℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2SO4; benzene View Scheme | |
| Multi-step reaction with 2 steps 1: toluene / 4 h / Reflux 2: toluene / 20 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | |
| With ammonia In water for 0.0166667h; | |
| With sodium hydroxide In water at 20℃; for 2h; |
-
-
1122-91-4
4-bromo-benzaldehyde

-
-
100-58-3
phenylmagnesium bromide

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| In diethyl ether for 5h; Reflux; |
-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
776-74-9
Bromodiphenylmethane

-
A
-
91-01-0
1,1-Diphenylmethanol

-
B
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
C
-
574-42-5
benzhydryl ether

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at 100℃; under 12929 Torr; for 0.0833333h; Overall yield = 48 %Spectr.; | |
| In 1-methyl-pyrrolidin-2-one at 100℃; under 12929 Torr; for 0.0833333h; Overall yield = 7 %Spectr.; |

-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
776-74-9
Bromodiphenylmethane

-
A
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
B
-
574-42-5
benzhydryl ether

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at 100℃; under 12929 Torr; for 0.0833333h; Overall yield = 77 %Spectr.; |

-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
90-99-3
diphenylchloromethane

-
A
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
B
-
574-42-5
benzhydryl ether

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 200℃; under 12929 Torr; for 0.266667h; Overall yield = 78 %Spectr.; |

-
-
64-18-6
formic acid

-
-
17471-10-2
Desmethyldiphenhydramine

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex; 1,3-bis-(diphenylphosphino)propane; phenylsilane In dibutyl ether at 60℃; for 18h; Schlenk technique; Inert atmosphere; | 92 %Chromat. |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium tert-butylate / tetrahydrofuran / 12 h / 20 °C 2: toluene-4-sulfonic acid / 36 h / 60 °C View Scheme |
-
-
27058-12-4
DMAE benzoate

-
-
24388-23-6
2-phenyl-4,4,5,5-tetramethyl-1,3,2-dioxoborole

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; cobalt acetylacetonate at 80℃; for 48h; Temperature; | 9.6 mmol |
-
-
27058-12-4
DMAE benzoate

-
-
98-80-6
phenylboronic acid

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; nickel(II) acetylacetonate In toluene at 70℃; for 48h; Temperature; | 9.6 mmol |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: magnesium / 0.67 h / Reflux; Inert atmosphere 1.2: 5 h / Reflux; Inert atmosphere 2.1: toluene / 4 h / Reflux 3.1: toluene / 20 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: magnesium / 0.67 h / Reflux; Inert atmosphere 1.2: 5 h / Reflux; Inert atmosphere 2.1: toluene / 4 h / Reflux 3.1: toluene / 20 h / Reflux View Scheme |
-
-
27550-73-8
Chloromethyl methacrylate

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
-
76637-18-8
(2-Benzhydryloxy-ethyl)-dimethyl-(2-methyl-acryloyloxymethyl)-ammonium; chloride

| Conditions | Yield |
|---|---|
| In acetone | 80% |
-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
-
90818-60-3
7-(2-bromoethyleoxy)benzopyran-2-one

| Conditions | Yield |
|---|---|
| In acetone at 70℃; for 6h; | 77% |

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With 2-Picolinic acid; iron(III) chloride; tert-Butyl peroxybenzoate; 18-crown-6 ether In acetonitrile at 50℃; for 48h; | A 54% B 27% |

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
-
21085-72-3
1-bromo-2,3,4-tri-O-acetyl-α-D-glucuronic acid methyl ester

-
-
145823-15-0
(2-Benzhydryloxy-ethyl)-((2R,3R,4S,5S,6S)-6-carboxy-3,4,5-trihydroxy-tetrahydro-pyran-2-yl)-dimethyl-ammonium; chloride

| Conditions | Yield |
|---|---|
| With XAD-2 ion-exchange resin; sodium hydrogencarbonate In water; benzene for 72h; Ambient temperature; | 32% |
-
-
74-83-9
methyl bromide

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
-
31065-89-1
(2-benzhydryloxy-ethyl)-trimethyl-ammonium; bromide

| Conditions | Yield |
|---|---|
| With ethanol |
-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
-
535-11-5, 41978-69-2
Ethyl 2-bromopropionate

| Conditions | Yield |
|---|---|
| With di-isopropyl ether |

| Conditions | Yield |
|---|---|
| With ethyl acetate |

| Conditions | Yield |
|---|---|
| With ethyl acetate |

| Conditions | Yield |
|---|---|
| With ethyl acetate |
-
-
927-68-4
bromoethyl acetate

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
-
38697-55-1
(2-acetoxy-ethyl)-(2-benzhydryloxy-ethyl)-dimethyl-ammonium; bromide

| Conditions | Yield |
|---|---|
| With butanone |

| Conditions | Yield |
|---|---|
| With benzene |
-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

-
-
140-18-1
Benzyl chloroacetate

-
-
6322-72-1
(2-benzhydryloxy-ethyl)-benzyloxycarbonylmethyl-dimethyl-ammonium; chloride

| Conditions | Yield |
|---|---|
| With benzene |

| Conditions | Yield |
|---|---|
| With toluene |

| Conditions | Yield |
|---|---|
| With sodium; toluene |

| Conditions | Yield |
|---|---|
| With ethyl acetate |

| Conditions | Yield |
|---|---|
| With ethyl acetate |

| Conditions | Yield |
|---|---|
| With ethyl acetate |

| Conditions | Yield |
|---|---|
| With benzene |

| Conditions | Yield |
|---|---|
| In benzene |
-
-
48073-23-0
1,10-bis-chloroacetoxy-decane

-
-
58-73-1
2-diphenylmethoxy-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| In benzene |
Diphenhydramine Consensus Reports
Reported in EPA TSCA Inventory.
Diphenhydramine Specification
The Diphenhydramine, with the CAS registry number 58-73-1, is also known as N-[2-(Diphenylmethoxy)ethyl]-N,N-dimethylamine. Its EINECS number is 200-396-7. This chemical's molecular formula is C17H21NO and molecular weight is 255.35. What's more, its systematic name is 2-(Diphenylmethoxy)-N,N-dimethylethanamine. Its classification codes are: (1)Anesthetics; (2)Anesthetics, local; (3)Anti-allergic agents; (4)Antiemetics; (5)Autonomic Agents; (6)Central Nervous System Agents; (7)Central Nervous System Depressants; (8)Drug / Therapeutic Agent; (9)Gastrointestinal Agents; (10)Histamine Agents; (11)Histamine Antagonists; (12)Histamine H1 antagonists; (13)Human Data; (14)Hypnotics and Sedatives; (15)Mutation data; (16)Neurotransmitter Agents; (17)Peripheral Nervous System Agents; (18)Reproductive Effect; (19)Sensory System Agents. This chemical is a first generation (typical) antihistamine used to treat a number of conditions including: allergic symptoms and itchiness, the common cold, insomnia, motion sickness, and extrapyramidal symptoms. It has a histamine H1 antagonist used as an antiemetic, antitussive, for dermatoses and pruritus, for hypersensitivity reactions, as a hypnotic, an antiparkinson, and as an ingredient in common cold preparations. It has some undesired antimuscarinic and sedative effects.
Physical properties of Diphenhydramine are: (1)ACD/LogP: 2.997; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.13; (4)ACD/LogD (pH 7.4): 1.63; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 4.78; (7)ACD/KOC (pH 5.5): 1.37; (8)ACD/KOC (pH 7.4): 43.56; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 6; (12)Polar Surface Area: 12.47 Å2; (13)Index of Refraction: 1.551; (14)Molar Refractivity: 79.568 cm3; (15)Molar Volume: 249.226 cm3; (16)Polarizability: 31.543×10-24cm3; (17)Surface Tension: 38.67 dyne/cm; (18)Density: 1.025 g/cm3; (19)Flash Point: 101.53 °C; (20)Enthalpy of Vaporization: 58.762 kJ/mol; (21)Boiling Point: 343.734 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
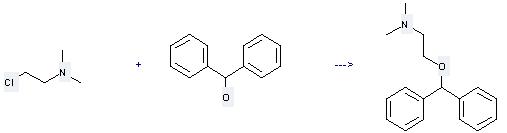
Preparation: this chemical can be prepared by (2-chloro-ethyl)-dimethyl-amine and diphenylmethanol at the room temperature. This reaction will need reagent NaH and solvent benzene with the reaction time of 1.5 hours. The yield is about 92%.
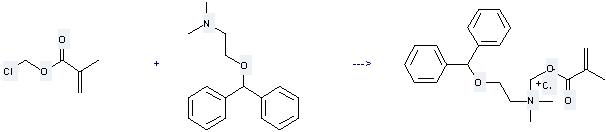
Uses of Diphenhydramine: it can be used to produce C22H28NO3(1+)·Cl(1-). It will need reagent acetone. The yield is about 80%.
You can still convert the following datas into molecular structure:
(1)SMILES: O(CCN(C)C)C(c1ccccc1)c2ccccc2
(2)Std. InChI: InChI=1S/C17H21NO/c1-18(2)13-14-19-17(15-9-5-3-6-10-15)16-11-7-4-8-12-16/h3-12,17H,13-14H2,1-2H3
(3)Std. InChIKey: ZZVUWRFHKOJYTH-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | intraperitoneal | 75mg/kg (75mg/kg) | Therapie. Vol. 28, Pg. 767, 1973. | |
| guinea pig | LD50 | subcutaneous | 56mg/kg (56mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 4, Pg. 189, 1954. | |
| human | TDLo | oral | 714ug/kg (.714mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ALTERATION OF OPERANT CONDITIONING BEHAVIORAL: CHANGES IN PSYCHOPHYSIOLOGICAL TESTS | Drug Development Research. Vol. 27, Pg. 33, 1992. |
| man | LDLo | unreported | 7353ug/kg (7.353mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| mouse | LD50 | intraperitoneal | 56mg/kg (56mg/kg) | Yakkyoku. Pharmacy. Vol. 34, Pg. 27, 1983. | |
| mouse | LD50 | intravenous | 29mg/kg (29mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Russian Pharmacology and Toxicology Vol. 40, Pg. 42, 1977. |
| mouse | LD50 | oral | 160mg/kg (160mg/kg) | Chimica Therapeutica. Vol. 7, Pg. 224, 1972. | |
| mouse | LD50 | subcutaneous | 50mg/kg (50mg/kg) | Bollettino Chimico Farmaceutico. Vol. 111, Pg. 293, 1972. | |
| rat | LD50 | intraperitoneal | 280mg/kg (280mg/kg) | Indian Journal of Medical Research. Vol. 59, Pg. 614, 1971. | |
| rat | LD50 | intravenous | 42mg/kg (42mg/kg) | Drugs in Japan Vol. -, Pg. 476, 1990. | |
| rat | LD50 | oral | 390mg/kg (390mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Russian Pharmacology and Toxicology Vol. 40, Pg. 42, 1977. |
| rat | LD50 | subcutaneous | 474mg/kg (474mg/kg) | Drugs in Japan Vol. -, Pg. 476, 1990. | |
| women | TDLo | multiple routes | 793ug/kg/1H-I (.793mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) BEHAVIORAL: ATAXIA BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" | Pharmacotherapy Vol. 14, Pg. 492, 1994. |
| women | TDLo | oral | 30mg/kg (30mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP VASCULAR: BP ELEVATION NOT CHARACTERIZED IN AUTONOMIC SECTION | Deutsche Medizinische Wochenschrift. Vol. 120, Pg. 1695, 1995. |
| women | TDLo | oral | 82mg/kg (82mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: COMA | Toxicology Letters. Vol. 109(Suppl, |
| women | TDLo | oral | 178mg/kg (178mg/kg) | CARDIAC: EKG CHANGES NOT DIAGNOSTIC OF ABOVE VASCULAR: BP ELEVATION NOT CHARACTERIZED IN AUTONOMIC SECTION BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Annals of Emergency Medicine. Vol. 33, Pg. 104, 1999. |
Related Products
- Diphenhydramine
- Diphenhydramine citrate
- Diphenhydramine hydrochloride
- 5873-16-5
- 587-33-7
- 5873-43-8
- 58734-57-9
- 5873-51-8
- 5873-57-4
- 58737-22-7
- 58738-31-1
- 5873-90-5
- 5873-93-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View