-
Name
DL-Alanine
- EINECS 206-126-4
- CAS No. 302-72-7
- Article Data259
- CAS DataBase
- Density 1.161 g/cm3
- Solubility 156 g/L (20 °C) in water
- Melting Point 289 °C (dec.)(lit.)
- Formula C3H7NO2
- Boiling Point 212.907 °C at 760 mmHg
- Molecular Weight 89.0941
- Flash Point 82.563 °C
- Transport Information
- Appearance White crystalline powder
- Safety 22-24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms (R,S)-Alanine;dl-.alpha.-Aminopropionic acid;DL-alpha-Alanine;D,L-Alanine;Alanin;DL-2-Aminopropanoic acid;(+-)-2-Aminopropionic acid;alanine;(RS)-2-Aminopropionsaeure;Alanine, DL-;(+-)-Alanine;DL-2-Aminopropionic acid;DL-.alpha.-Alanine;D, L-Alanine;DL-alpha-Aminopropionic acid;H-DL-Ala-OH;DL-2-Aminopropanic Acid,;
- PSA 63.32000
- LogP 0.11850
Synthetic route

| Conditions | Yield |
|---|---|
| With salicylaldehyde In acetic acid at 100℃; for 1h; Product distribution; var. temp., var. catalysts, var. solvents; | 96% |
| salicylaldehyde In acetic acid at 100℃; for 1h; | 96% |
| With sodium dihydrogenphosphate; tritium oxide at 124.6 - 175.4℃; Thermodynamic data; Rate constant; various pH's, various temperatures; Ea, ΔH(excit.), ΔS(excit.); |
-
-
100-39-0
benzyl bromide

-
-
117470-83-4
ethyl N-<(4-chlorophenyl)methylene>-DL-alanate

-
A
-
1132-26-9
2-methyl-3-phenylalanine

-
B
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In acetonitrile for 48h; Product distribution; Heating; further alkylation method; | A 94% B 7% |


| Conditions | Yield |
|---|---|
| With phenylglycin In water; acetonitrile for 24h; Inert atmosphere; Reflux; | 90% |
| With ethylenediaminetetraacetic acid; cetyltrimethylammonium chloride; N-Phenylglycine; DPL at 30℃; Product distribution; potassium phosphate buffer (pH 7.8); variation of incubation time, pH and DPL concentration; | |
| With zinc(II) perchlorate; 15-aminomethyl-14-hydroxy-5,5-dimethyl-2,8-dithia<9>(2,5)pyridinophane In methanol at 23℃; Rate constant; |

| Conditions | Yield |
|---|---|
| With sodium dithionite; sodium carbonate In acetonitrile | 90% |
-
-
80222-96-4
2-aminopropanamide hydrochloride

-
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopropanamide hydrochloride With sodium hydroxide In water Stage #2: In water at 130℃; for 1h; | 88.6% |

| Conditions | Yield |
|---|---|
| With phenylglycin In water; acetonitrile for 24h; Inert atmosphere; Reflux; | 88% |
-
-
120-20-7
2-(3,4-dimethoxyphenyl)-ethylamine

-
-
4192-28-3
Phth-L-Ala-OH

-
A
-
26477-09-8
N-2-(3,4-dimethoxyphenyl)ethylphthalimide

-
B
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| In methanol at 140 - 145℃; for 0.666667h; Solvent; | A 78% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: sodium pyruvate With ammonia; hydrogen; chloro(1,5-cyclooctadiene)rhodium(I) dimer; 2,2'-bis[[bis(3-sulfophenyl)phosphino]methyl]-4,4',7,7'-tetrasulfo-1,1'-binaphthyl octasodium salt In water at 60℃; under 24752.5 Torr; for 16h; Stage #2: With hydrogenchloride In water Product distribution / selectivity; | 75% |
| With copper(II) perchlorate; 2-methyl-3-hydroxy-4-aminomethyl-5-(dodecylthiomethyl)pyridine In water at 30℃; | |
| Multistep reaction; | |
| With 2-methyl-3-hydroxy-4-aminomethyl-5-(N,N-didodecylamino)pyridine hydrobromide; N,N-hexadecyl-Nα-<6-(triethylammonio)hexanoyl>-L-alaninamide bromide; copper(II) perchlorate In water at 9.9℃; Thermodynamic data; Rate constant; Mechanism; pH = 6.9, (HEPES buffer), transamination; +ΔG, +ΔH (depends quant. of cat.), var. temp.; | |
| With copper(II) perchlorate; 2-methyl-3-hydroxy-4-aminomethyl-5-(dodecylthiomethyl)pyridine In water at 30℃; Rate constant; Mechanism; various vesicle-forming compound added; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Amino-1-propanol With (6-di-tert-butylphosphinomethyl-2,2’-bipyridyl)Ru(CO)HCl; water; sodium hydroxide In 1,4-dioxane for 24h; Schlenk technique; Inert atmosphere; Glovebox; Reflux; Stage #2: With hydrogenchloride In 1,4-dioxane; water | 71% |
| With potassium permanganate; sulfuric acid |
-
-
100-39-0
benzyl bromide

-
-
69555-16-4
ethyl 2-(diphenylmethyleneamino)propanoate

-
A
-
1132-26-9
2-methyl-3-phenylalanine

-
B
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In acetonitrile for 48h; Product distribution; Heating; further alkylation method; | A 23% B 63% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether Yield given; | A 63% B n/a |
| With hydrogenchloride In diethyl ether Yields of byproduct given; | A 63% B n/a |
-
-
77504-36-0
2-amino-3-((4-((S)-2-amino-2-carboxyethyl)-1H-imidazol-2-yl)thio)propionic acid

-
A
-
302-72-7
rac-Ala-OH

-
B
-
2002-22-4
(2S)-2-amino-3-(2-mercapto-1H-imidazol-4-yl)propanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen iodide for 16h; Heating; | A 54% B 62% |
-
-
130642-14-7, 130642-18-1
Acetic acid (2R,4R)-2,4-dimethyl-3-nitro-5-oxo-oxazolidin-2-yl ester

-
A
-
60-35-5
acetamide

-
B
-
631-61-8
ammonium acetate

-
C
-
130642-15-8
N-nitroalaninamide

-
D
-
515-98-0
Ammonium lactate

-
E
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether at 25℃; for 0.5h; Mechanism; | A n/a B n/a C n/a D n/a E 60% |

-
-
130642-14-7, 130642-18-1
Acetic acid (2R,4R)-2,4-dimethyl-3-nitro-5-oxo-oxazolidin-2-yl ester

-
A
-
60-35-5
acetamide

-
B
-
130642-15-8
N-nitroalaninamide

-
C
-
515-98-0
Ammonium lactate

-
D
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether at 25℃; for 0.5h; Further byproducts given; | A n/a B 60% C n/a D n/a |


-
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; 2.5% Ru on PICA 12x20 mesh granular C; ammonium metatungstate hydrate at 180℃; under 103510 Torr; for 2h; Pressure; Reagent/catalyst; Temperature; Sealed tube; | 56.5% |

-
A
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether Yield given; | A n/a B 55% |
| With hydrogenchloride In diethyl ether | A n/a B 55% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether | A 51% B n/a |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 70℃; for 5h; | 46% |
-
-
598-78-7
(R,S)-2-chloropropionic acid

-
A
-
73890-66-1, 219755-19-8
2,2'-iminodipropionic acid

-
B
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 70℃; for 5h; Product distribution; var. initial ratios of reagents and temperatures and pressures; | A n/a B 46% |

-
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetic acid at 100℃; for 48h; biomimetic reduction; | 34% |
-
-
39484-07-6
2-Amino-3-(2,5-dihydroxy-phenylsulfanyl)-propionic acid

-
A
-
123-31-9
hydroquinone

-
B
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| In methanol for 1h; Irradiation; | A 32% B 20% |
-
-
63-91-2
L-phenylalanine

-
-
113-24-6
sodium pyruvate

-
A
-
114-76-1
sodium phenylphyruvate

-
B
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With acetate buffer; N(1+)C5His2C16; PL(1+)2C16; copper(II) perchlorate In water at 30℃; for 48h; Kinetics; var. catalysts; | A n/a B 27% |

-
-
17054-97-6
3-S-cysteinyl-5-methylcatechol

-
A
-
302-72-7
rac-Ala-OH

-
B
-
452-86-8
4-methyl-1,2-dihydroxybenzene

| Conditions | Yield |
|---|---|
| In methanol for 1h; Product distribution; Mechanism; Irradiation; further in presence of O2, acetone, or 2-methyl-1,3-butadiene; | A 20% B 16% |
-
-
107-10-8
propylamine

-
-
64-18-6
formic acid

-
A
-
10569-72-9
DL-3-aminoisobutyric acid

-
B
-
302-72-7
rac-Ala-OH

-
C
-
2835-81-6
2-aminobutanoic acid

-
D
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; contact glow discharge electrolysis (500-600 V, 45 mA); Further byproducts given; | A 9.8% B 3.4% C 0.9% D 8.1% |


| Conditions | Yield |
|---|---|
| With nitrogen; water at 95℃; under 4560.31 Torr; for 72h; Catalytic behavior; Pressure; UV-irradiation; | A 3.4% B 2.4% |
| With nitrogen; water Reagent/catalyst; UV-irradiation; Heating; |

-
-
64-18-6
formic acid

-
-
75-04-7
ethylamine

-
A
-
302-84-1
serin

-
B
-
56-40-6
glycine

-
C
-
302-72-7
rac-Ala-OH

-
D
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; contact glow discharge electrolysis (500-600 V, 45 mA); | A 0.1% B 0.1% C 1.5% D 3.1% |

-
-
141-53-7
sodium formate

-
-
75-04-7
ethylamine

-
A
-
302-84-1
serin

-
B
-
56-40-6
glycine

-
C
-
302-72-7
rac-Ala-OH

-
D
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; contact glow discharge electrolysis (500-600 V, 45 mA); | A 1% B 0.3% C 3.1% D 0.6% |


-
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| at 19.9℃; under 37503000 Torr; Product distribution; under shear deformation (360 deg); | 1.35% |

| Conditions | Yield |
|---|---|
| With diethyl ether Erhitzen des Reaktionsprodukts mit konz. wss. HCl auf 90grad; |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 20℃; for 17h; Inert atmosphere; | 100% |
| With thionyl chloride at -20 - 20℃; for 17h; Inert atmosphere; | 100% |
| With chloro-trimethyl-silane at 20℃; for 12h; | 97% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at -20℃; for 4h; Heating / reflux; | 100% |
| With thionyl chloride for 16h; Heating; |

| Conditions | Yield |
|---|---|
| at 200℃; for 0.05h; Product distribution / selectivity; Microwave irradiation (300 W); | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 72h; | 99.99% |

| Conditions | Yield |
|---|---|
| In ethanol for 36h; Esterification; Heating; | 99% |
-
-
82704-14-1
(S)-2-{(N-benzyl-2-pyrrolidinyl)carbonylamino}benzaldehyde

-
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol | 99% |


| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol at 65℃; diastereoselective reaction; | 99% |

-
-
7149-49-7
2,3-naphthalenedicarboxaldehyde

-
-
143-33-9
sodium cyanide

-
-
302-72-7
rac-Ala-OH

-
-
103851-04-3
Sodium; 2-(1-cyano-benzo[f]isoindol-2-yl)-propionate

| Conditions | Yield |
|---|---|
| In methanol; water for 0.75h; Ambient temperature; | 98.6% |

-
-
302-72-7
rac-Ala-OH

-
-
2719-27-9
cyclohexanylcarbonyl chloride

-
-
150671-92-4
N-(Cyclohexylcarbonyl)-DL-alanin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane at 20℃; for 0.5h; | 98% |
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; butan-1-ol Ambient temperature; | 98% |
-
-
91806-74-5
3,5-dimethyl-pyrazole-1-carboxylic acid benzyl ester

-
-
302-72-7
rac-Ala-OH

-
-
4132-86-9, 26607-51-2, 1142-20-7
N-benzyloxycarbonylalanine

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at -5℃; for 5h; | 98% |
-
-
909114-65-4
tert-butyl 4,6-dimethoxy-1,3,5-triazinyl carbonate

-
-
302-72-7
rac-Ala-OH

-
-
3744-87-4, 7764-95-6, 15761-38-3
N-t-butyloxycarbonyl-DL-alanine

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 0.5h; | 98% |
-
-
1644308-43-9
(R)-N-(2-benzoyl-4-chlorophenyl)-1-[(3,4-dichlorophenyl)methyl]-2-pyrrolidinecarboxamide

-
-
6018-89-9
nickel(II) acetate tetrahydrate

-
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 60℃; for 1h; Temperature; diastereoselective reaction; | 98% |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Reflux; | 97.56% |
-
-
701-73-5
methyl N-phenyldithiocarbamate

-
-
302-72-7
rac-Ala-OH

-
-
4333-19-1
5-methyl-3-phenyl-2-thiohydantoin

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 5h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With thionyl chloride for 2h; Heating; | 97% |
| With hydrogenchloride for 5h; Heating; | 95% |
| With hydrogenchloride for 5h; Reflux; | 92% |
-
-
1644308-41-7
((S)-N-(2-benzoyl-4-chlorophenyl)-1-(3,4-dichlorobenzyl)pyrrolidine-2-carboxamide)

-
-
6018-89-9
nickel(II) acetate tetrahydrate

-
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 60℃; for 3h; Temperature; Reagent/catalyst; diastereoselective reaction; | 97% |
| With potassium carbonate In methanol at 15℃; for 56h; | 90% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 6h; Reflux; | 97% |

| Conditions | Yield |
|---|---|
| With acetic acid at 100℃; for 3h; | 96.4% |
-
-
58303-62-1
5-(4-chlorophenyl)-2,3-dihydrofuran-2,3-dione

-
-
302-72-7
rac-Ala-OH

-
-
121275-51-2
2-[4-(4-Chloro-phenyl)-2,4-dioxo-butyrylamino]-propionic acid

| Conditions | Yield |
|---|---|
| With acetamide at 78 - 80℃; for 0.416667h; oth. reagent - CH3CON(CH3)2; | 96% |
-
-
909114-66-5
9-fluorenylmethyl 4,6-dimethoxy-1,3,5-triazinyl carbonate

-
-
302-72-7
rac-Ala-OH

-
-
35661-38-2
Fmoc-alanine

| Conditions | Yield |
|---|---|
| With sodium carbonate In acetonitrile at 20℃; for 1.25h; | 96% |
-
-
1131147-58-4
3-(1H-benzo[d][1,2,3]triazole-1-carbonyl)-1-ethyl-7-methyl-1,8-naphthyridin-4(1H)-one

-
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With triethylamine In water; acetonitrile at 20℃; for 3h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: Benzoyl isothiocyanate; rac-Ala-OH With 1-n-butyl-3-methylimidazolim bromide at 50℃; for 1h; Stage #2: ethyl Bromopyruvate at 50℃; for 3h; | 96% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 60 - 70℃; for 24h; | 96% |
| With potassium carbonate In methanol at 40℃; for 24h; | 84.8% |
| With potassium carbonate In methanol at 40℃; for 24h; | 84.8% |

-
-
79-11-8
chloroacetic acid

-
-
302-72-7
rac-Ala-OH

-
-
164462-16-2
N,N-bis(carboxymethyl)-DL-alanine trisodium salt

| Conditions | Yield |
|---|---|
| Stage #1: rac-Ala-OH With sodium carbonate; sodium iodide In water at 55℃; for 1.08333h; pH=9.5 - 9.9; Large scale; Stage #2: chloroacetic acid In water at 45 - 92℃; Reagent/catalyst; Large scale; | 96% |

| Conditions | Yield |
|---|---|
| In water at 37℃; for 10h; NADH, ammonium chloride, sodium formate, D-amino acid oxidase, catalase, leucine dehydrogenase, formate dehydrogenase, Tris-HCl buffer, pH 8.5; | 95% |
| optische Spaltung; | |
| With D-amino acid oxide ase-substance |
-
-
501-53-1
benzyl chloroformate

-
-
302-72-7
rac-Ala-OH

-
-
4132-86-9, 26607-51-2, 1142-20-7
N-benzyloxycarbonylalanine

| Conditions | Yield |
|---|---|
| With sodium carbonate In tetrahydrofuran; water at 0 - 20℃; for 2h; | 95% |
| With sodium hydroxide at 0℃; for 3h; | 68% |
| With sodium hydroxide unter Kuehlung; |

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethylguanidine | 95% |
| Stage #1: rac-Ala-OH With triethylamine In methanol for 0.0833333h; Stage #2: ethyl trifluoroacetate, In methanol for 24h; | 68% |
| With methanol; ion-exchange resin + form>; N-cyclohexyl-cyclohexanamine | |
| With triethylamine In methanol at 20℃; for 16h; |
-
-
302-72-7
rac-Ala-OH

-
-
407-25-0
trifluoroacetic anhydride

-
-
2546-67-0
4-Methyl-2-(trifluoromethyl)-1,3-oxazol-5(2H)-on

| Conditions | Yield |
|---|---|
| at 20℃; for 4h; | 95% |
| at 150℃; for 2h; | 64% |
| at 0 - 60℃; for 2h; | 41% |
Dl-Alanine Chemical Properties
IUPAC Name: 2-aminopropanoic acid
Synonyms of Dl-Alanine (CAS NO.302-72-7): (+-)-2-Aminopropionic acid ; (+-)-Alanine ; (R,S)-Alanine ; Alanine, DL- ; D,L-Alanine ; DL-2-Aminopropanoic acid ; DL-alpha-Aminopropionic acid
CAS NO: 302-72-7
Molecular Formula: C3H7NO2
Molecular Weight: 89.09
Molecular Structure: 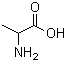
EINECS: 206-126-4
H bond acceptors: 3
H bond donors: 3
Freely Rotating Bonds: 2
Polar Surface Area: 29.54 Å2
Index of Refraction: 1.459
Molar Refractivity: 21 cm3
Molar Volume: 76.7 cm3
Surface Tension: 45.8 dyne/cm
Density: 1.161 g/cm3
Flash Point: 82.6 °C
Enthalpy of Vaporization: 49.5 kJ/mol
Boiling Point: 212.9 °C at 760 mmHg
Vapour Pressure: 0.0661 mmHg at 25°C
Melting Point: 289 °C (dec.)(lit.)
Storage temp: Store at RT.
Water Solubility: 156 g/L (20 °C)
Appearance: White crystalline powder
Stability: Stable. Incompatible with strong oxidizing agents.
Product Categories of Dl-Alanine (CAS NO.302-72-7): Amino Acids;Alanine [Ala, A];alpha-Amino Acids;Biochemistry;Amino Acids;alanine;amino acid;bio-chemical;chemicals;food additive;food additives;food flavor;organic acids;pharmaceutical intermediate;sweetener
Dl-Alanine Uses
Dl-Alanine (CAS NO.302-72-7) is a biologically important amino acid.
Dl-Alanine Production
D-Alanine occurs in bacterial cell walls and in some peptide antibiotics.
Here is Chemical Synthesis:
D-Alanine can be prepared by the condensation of acetaldehyde with ammonium chloride in the presence of sodium cyanide by the Strecker reaction, or by the ammonolysis of 2-bromopropionic acid
.png)
Dl-Alanine Safety Profile
Safety Information about Dl-Alanine (CAS NO.302-72-7):
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 22-24/25-36-26
S22: Do not breathe dust.
S24/25: Avoid contact with skin and eyes.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36: Wear suitable protective clothing.
WGK Germany: 3
RTECS: AY2980000
HS Code: 29224995
Low toxicity by ingestion. When heated to decomposition emits toxic fumes of NOx.
Dl-Alanine Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam.
Handling: Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Storage: Store in a cool, dry place. Store in a tightly closed container. Do not exceed 30°C (86°F).
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View