-
Name
O-PHOSPHORYLETHANOLAMINE
- EINECS
- CAS No. 1071-23-4
- Article Data34
- CAS DataBase
- Density 1.554g/cm3
- Solubility Soluble in water
-
Melting Point
241-243 °C(lit.)
- Formula C2H8 N O4 P
- Boiling Point 335.8°Cat760mmHg
- Molecular Weight 141.064
- Flash Point 156.9°C
- Transport Information
- Appearance white powder
- Safety Moderately toxic by intravenous route. When heated to decomposition it emits very toxic fumes of POx and NOx.
- Risk Codes 25-34
-
Molecular Structure
-
Hazard Symbols

- Synonyms Ethanol,2-amino-, dihydrogen phosphate (ester) (8CI,9CI); Ethanol, 2-amino-, phosphate(6CI); Phosphoric acid, 2-aminoethyl ester (6CI); 2-Aminoethanol O-phosphate;2-Aminoethyl dihydrogen phosphate; Colamine phosphate; EthanolamineO-phosphate; Mono(2-aminoethyl) phosphate; Monoaminoethyl phosphate; NSC254167; O-Phosphoethanolamine; O-Phosphorylethanolamine; Phosphoethanolamine;Phosphonoethanolamine; Phosphorylethanolamine
- PSA 102.59000
- LogP -0.24530
Synthetic route

| Conditions | Yield |
|---|---|
| With phosphoric acid at 100℃; under 10 Torr; for 3h; | 60% |
| With phosphoric acid 1) 100 deg C, 10 mmHg, 1 h; 2) 150 deg C, 0.3 mmHg, 3 h; | 60% |
| With phosphoric acid at 185℃; for 8h; Cooling with ice; | 45% |

| Conditions | Yield |
|---|---|
| With phosphoric acid at 105℃; |

| Conditions | Yield |
|---|---|
| With phosphoric acid at 165℃; |

| Conditions | Yield |
|---|---|
| With ammonia; water at 120 - 130℃; |
-
-
14662-76-1
(2-hydroxy-ethyl)-amidophosphoric acid diisopropyl ester

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
102313-50-8
(2-diphenoxyphosphoryloxy-ethyl)-carbamic acid benzyl ester

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| With ethanol; palladium Hydrogenation.anschliessend an Platin in Essigsaeure; |

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate Gegenwart von alkalischer Phosphatase bei pH 9 und 37grad; |

| Conditions | Yield |
|---|---|
| at 130℃; |
-
-
65314-76-3
phosphoric acid-(2-amino-ethyl ester)-diphenyl ester

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate
-
-
102313-50-8
(2-diphenoxyphosphoryloxy-ethyl)-carbamic acid benzyl ester

-
-
64-19-7
acetic acid

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| Hydrogenation; |


| Conditions | Yield |
|---|---|
| at 37℃; pH=7.4; Kinetics; Equilibrium constant; aq. phosphate buffer; |

-
A
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
B
-
3163-37-9, 51534-41-9, 22644-96-8
(E)-hexadec-2-enal

| Conditions | Yield |
|---|---|
| With sphingosine-1-phosphate lyase Enzymatic reaction; |
-
-
26993-30-6
Sphingosine-1-phosphate

-
A
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
B
-
3163-37-9, 51534-41-9, 22644-96-8
(E)-hexadec-2-enal

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate; human sphingosine-1-phosphate lyase In water; dimethyl sulfoxide at 20℃; for 22h; pH=8; Kinetics; Reagent/catalyst; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| In water for 4h; | 99% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
1257405-75-6
azidomethyl 4-nitrophenyl carbonate

-
-
1257405-79-0
ammonium 2-[(azidomethoxy)carbonylamino]ethylhydrogenphosphate

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethyl dihydrogen phosphate; azidomethyl 4-nitrophenyl carbonate With water; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: With water; ammonium bicarbonate | 99% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
144-55-8
sodium hydrogencarbonate

-
-
4055-39-4
mitomycin A

-
-
18623-11-5
octadecyl silane

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; phosphorus pentaoxide; water | 97% |

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide

-
-
329223-23-6
(2-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}ethoxy)phosphonic acid

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 20℃; for 1h; | 89% |
| With water; sodium carbonate In N,N-dimethyl-formamide for 0.333333h; | 75% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
28920-43-6
(fluorenylmethoxy)carbonyl chloride

-
-
329223-23-6
(2-{[(9H-fluoren-9-ylmethoxy)carbonyl]amino}ethoxy)phosphonic acid

| Conditions | Yield |
|---|---|
| Stage #1: chloro-trimethyl-silane; 2-aminoethyl dihydrogen phosphate In dichloromethane for 2h; Heating; Stage #2: (fluorenylmethoxy)carbonyl chloride With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 6h; Further stages.; | 85% |

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
932014-80-7
(2-phosphonooxy-ethyl)-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; water at 20℃; for 17h; | 82% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| In water treated suspension of ZnO in H2O with 2-aminoethyl dihydrogen phosphate; stirring for 10 min at room temp.; filtration; slowly evapn. at 45°C; elem. anal.; | 81% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; pH=Ca.5; Stage #2: chloroacetyl chloride With lithium hydroxide In water pH=Ca.5; | 81% |
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; Stage #2: chloroacetyl chloride In water | 81% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethyl dihydrogen phosphate With potassium tert-butylate In methanol at 20℃; for 0.5h; Cooling with ice; Inert atmosphere; Stage #2: pyridoxal 5'-phosphate With potassium tert-butylate In methanol at 0 - 20℃; for 0.5h; Inert atmosphere; Stage #3: With sodium tetrahydroborate In methanol at 0℃; for 4h; Reflux; Darkness; Inert atmosphere; | 79% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; pH=Ca.5; Stage #2: monofluoroacetyl chloride With lithium hydroxide In water pH=Ca.5; | 78% |
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; Stage #2: monofluoroacetyl chloride In water | 78% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
1077-28-7, 62-46-4
Thioctic acid

-
-
1085381-76-5
2-(5-(1,2-dithiolan-3-yl)pentanamido)ethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| Stage #1: Thioctic acid With 1-hydroxy-pyrrolidine-2,5-dione; sodium hydrogencarbonate In water; N,N-dimethyl-formamide pH=7.4; Stage #2: 2-aminoethyl dihydrogen phosphate With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; sodium phosphate In water; N,N-dimethyl-formamide | 74% |

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane for 6h; Reflux; | 69% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
187224-28-8
(5Z,8Z,11Z,14Z)-2,5-dioxopyrrolidin-1-yl icosa-5,8,11,14-tetraenoate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran Ambient temperature; | 60% |
-
-
38020-81-4
2-iodoacetyl chloride

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; pH=Ca.5; Stage #2: 2-iodoacetyl chloride With lithium hydroxide In water pH=Ca.5; | 58% |
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; Stage #2: 2-iodoacetyl chloride In water | 58% |
-
-
3392-07-2
tert-butyl N-{2-[(2,5-dioxopyrrolidin-1-yl)oxy]-2-oxoethyl}carbamate

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In methanol at 20℃; for 3h; | 57% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
1425803-45-7
1,3-dioxoisoindolin-2-yl 2-(cyclooct-2-yn-1-yloxy)acetate

| Conditions | Yield |
|---|---|
| With triethylamine In water; N,N-dimethyl-formamide for 2h; | 54% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
598-21-0
2-Bromoacetyl bromide

-
-
52011-43-5
2-(2-bromoacetamido)ethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; pH=Ca.5; Stage #2: 2-Bromoacetyl bromide With lithium hydroxide In water pH=Ca.5; | 49% |
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; Stage #2: 2-Bromoacetyl bromide In water | 49% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
501-53-1
benzyl chloroformate

-
-
119220-16-5
dilithium 2-(benzyloxycarbonylamino)ethyl phosphate

| Conditions | Yield |
|---|---|
| With lithium hydroxide In benzene for 2h; | 47% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethyl dihydrogen phosphate With sodium hydrogencarbonate In dimethyl sulfoxide at 20℃; for 0.5h; Stage #2: chlorine e6 anhydride In dichloromethane; dimethyl sulfoxide at 20℃; Darkness; Stage #3: n-Octylamine In dichloromethane at 20℃; for 0.5h; | 44% |


| Conditions | Yield |
|---|---|
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; pH=Ca.5; Stage #2: phenylacetyl chloride With lithium hydroxide In water pH=Ca.5; | 32% |
| Stage #1: 2-aminoethyl dihydrogen phosphate With lithium hydroxide In water at 20℃; for 0.5h; Stage #2: phenylacetyl chloride In water | 32% |

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 80℃; under 7600.51 Torr; for 168h; Inert atmosphere; Autoclave; | 30% |

-
-
299969-20-3
2,5-dioxopyrrolidin-1-yl biphenyl-4-carboxylate

-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
1191406-80-0
N-(4-phenylbenzoyl)-2-aminoethyl phosphate

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; triethylamine In water; N,N-dimethyl-formamide at 20℃; for 3h; | 23% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
1191406-77-5
4-(N-benzylamino)benzoic acid N-hydroxysuccinimide ester

-
-
1191406-78-6
N-(4-(N-benzylamino)benzoyl)-2-aminoethyl phosphate

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; triethylamine In water; N,N-dimethyl-formamide at 20℃; for 3h; | 23% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; N,N-dimethyl-formamide at 20℃; for 24h; | 22% |
-
-
1071-23-4
2-aminoethyl dihydrogen phosphate

-
-
2946-39-6
8-bromoadenosine

-
-
1151916-63-0
3-[6-amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydroxymethyltetrahydrofuran-2-yl)-9H-purin-8-ylamino]-propionic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In water for 24h; Inert atmosphere; Reflux; | 14% |
ETHANOLAMINE PHOSPHATE Chemical Properties
Ethanolamine phosphate(1071-23-4)'s molecular formula is C2H8NO4P and its formula weight is 141.06.
Ethanolamine phosphate(1071-23-4) has a melting point of 241-243 °C(lit.).
Ethanolamine phosphate(1071-23-4) has the property of being dissolved in water. It is slightly soluble in methanol and ethanol.
The molecular structure of ethanolamine phosphate(1071-23-4):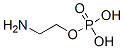
ETHANOLAMINE PHOSPHATE Uses
ETHANOLAMINE PHOSPHATE Toxicity Data With Reference
| 1. | ivn-mus LD50:639 mg/kg | RPOBAR Research Progress in Organic Biological and Medicinal Chemistry. 2 (1970),316. |
ETHANOLAMINE PHOSPHATE Consensus Reports
ETHANOLAMINE PHOSPHATE Safety Profile
ETHANOLAMINE PHOSPHATE Specification
Storage: Keep refrigerated. (Store below 4°C/39°F.) Store in a tightly closed container. Store in a dry area. Corrosives area.
Related Products
- Ethanolamine
- ETHANOLAMINE PHOSPHATE
- Ethanolamine thioglycolate
- 1071-27-8
- 107128-00-7
- 107-13-1
- 107133-36-8
- 107134-59-8
- 1071-37-0
- 1071-39-2
- 107-14-2
- 1071428-42-6
- 1071428-43-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View