-
Name
Elemicin
- EINECS 207-649-0
- CAS No. 487-11-6
- Article Data21
- CAS DataBase
- Density 1.011 g/cm3
- Solubility
- Melting Point
- Formula C12H16O3
- Boiling Point 279.8 °C at 760 mmHg
- Molecular Weight 208.257
- Flash Point 92.6 °C
- Transport Information
- Appearance Colorless oil-like liquid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Benzene,1,2,3-trimethoxy-5-(2-propenyl)- (9CI);Benzene, 5-allyl-1,2,3-trimethoxy-(8CI);Elemicin (6CI);1,2,3-Trimethoxy-5-(2-propenyl)benzene;1-(3,4,5-Trimethoxyphenyl)-2-propene;1-Allyl-3,4,5-trimethoxybenzene;3,4,5-Trimethoxyallylbenzene;4-Allyl-1,2,6-trimethoxybenzene;5-(Prop-2-enyl)-1,2,3-trimethoxybenzene;5-Allyl-1,2,3-trimethoxybenzene;NSC16704;
- PSA 27.69000
- LogP 2.44090
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 24h; | 96% |
| With potassium carbonate | |
| With potassium carbonate In acetone for 6h; Heating; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 16h; | 95% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 4.5h; | 93% |
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate In toluene at 110℃; for 15h; Suzuki-Miyaura coupling; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With pyridine; nickel(II) iodide; 4-chloro-2-(4,5-dihydro-1H-imidazol-2-yl)pyridine; tetrabutylammomium bromide; magnesium chloride; zinc In N,N-dimethyl acetamide at 60℃; for 12h; Reagent/catalyst; Temperature; Schlenk technique; Inert atmosphere; regioselective reaction; | 87% |
| With pyridine; nickel(II) iodide; tetrabutylammomium bromide; 4,4'-di-tert-butyl-2,2'-bipyridine; magnesium chloride; zinc In N,N-dimethyl acetamide at 60℃; for 12h; Inert atmosphere; | 79% |
-
-
2675-79-8
1-bromo-3,4,5-trimethoxybenzene

-
-
72824-04-5
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
487-11-6
elemicin

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); cesium fluoride In tetrahydrofuran at 85℃; for 24h; Suzuki-Miyaura coupling; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| In water; acetonitrile for 36h; Irradiation; | 54% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetone | |
| With potassium carbonate In acetone for 6h; Reflux; |

| Conditions | Yield |
|---|---|
| (i) (heating), (ii) KOH, EtOH, (iii) /BRN= 969135/; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With toluene; calcium carbonate Erhitzen auf Siedetemperatur; |
-
-
24313-88-0
3,4,5-Trimethoxyaniline

-
-
487-11-6
elemicin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 54 percent / acetonitrile; H2O / 36 h / Irradiation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 94 percent / K2CO3 / acetone / 24 h / 20 °C 2: 92 percent / 2 h / 200 °C 3: 96 percent / K2CO3 / acetone / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: K2CO3 / acetone 2: 2 h / 200 °C 3: K2CO3 View Scheme | |
| Multi-step reaction with 3 steps 1: K2CO3; acetone 2: 220 °C 3: NaOH-solution View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 92 percent / 2 h / 200 °C 2: 96 percent / K2CO3 / acetone / 24 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 2 h / 200 °C 2: K2CO3 View Scheme | |
| Multi-step reaction with 2 steps 1: 220 °C 2: NaOH-solution View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 2: alkaline aqueous H2O2 3: K2CO3; acetone View Scheme | |
| Multi-step reaction with 3 steps 1: hexan-1-amine / 6 h / Heating 2: dihydrogen peroxide; pyridine; sodium hydroxide / water / 1.5 h / 20 °C 3: potassium carbonate / acetone / 6 h / Reflux View Scheme |
-
-
22934-51-6
5-allyl-2-hydroxy-3-methoxybenzaldehyde

-
-
487-11-6
elemicin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: alkaline aqueous H2O2 2: K2CO3; acetone View Scheme |
-
-
214360-67-5
2-(3,4,5-trimethoxyphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
107-05-1
3-chloroprop-1-ene

-
-
487-11-6
elemicin

| Conditions | Yield |
|---|---|
| Stage #1: 4,4,5,5-tetramethyl-2-(3,4,5-trimethoxyphenyl)-1,3,2-dioxaborolane; 3-chloroprop-1-ene With bis(dibenzylideneacetone)-palladium(0) In methanol at 20℃; for 0.166667h; Inert atmosphere; Glovebox; Stage #2: With potassium fluoride In methanol at 20℃; for 24h; Inert atmosphere; Glovebox; | 140 mg |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 4,4'-di-tert-butyl-2,2'-bipyridine / tetrahydrofuran / 18 h / 80 °C / Inert atmosphere; Glovebox 2.1: bis(dibenzylideneacetone)-palladium(0) / methanol / 0.17 h / 20 °C / Inert atmosphere; Glovebox 2.2: 24 h / 20 °C / Inert atmosphere; Glovebox View Scheme |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In 1,2-dimethoxyethane at 80℃; Stille Cross Coupling; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: [bis(acetoxy)iodo]benzene / 0 - 20 °C 2: titanium tetrachloride / toluene; dichloromethane / 0.5 h / 0 °C 3: potassium carbonate / acetonitrile / 16 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With silver(l) oxide |
-
-
487-11-6
elemicin

-
-
487-12-7, 5273-84-7, 5273-85-8
isoelemicin

| Conditions | Yield |
|---|---|
| With nickel(II) iodide; 6,6'-dimethyl-2,2'-bipyridine; phosphonic acid diethyl ester; zinc In N,N-dimethyl acetamide at 35℃; for 24h; | 97% |
| With (μ-Cl)2Ni2(1,3-bis(2,6-diisopropylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene)2 In chlorobenzene at 20℃; for 3h; | 90% |
| With potassium hydroxide In ethanol | |
| With Grotjahn’s catalyst In [(2)H6]acetone at 20℃; for 0.333333h; Reagent/catalyst; Temperature; Gas phase; Inert atmosphere; diastereoselective reaction; | 98 %Spectr. |

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; triphenylphosphine; tert-butyl alcohol In tetrahydrofuran at 80℃; for 22h; Schlenk technique; Inert atmosphere; | 90% |
-
-
487-11-6
elemicin

-
-
69843-08-9
1-(1,1,1-trifluoroprop-2-en-2-yl)-4-methoxybenzene

| Conditions | Yield |
|---|---|
| With 6,6'-dimethyl-2,2'-bipyridine; nickel(II) bromide trihydrate; Triethoxysilane; tetrabutylammomium bromide; lithium fluoride; triphenylphosphine In N,N-dimethyl acetamide at 60℃; for 24h; Inert atmosphere; Sealed tube; chemoselective reaction; | 86% |
-
-
487-11-6
elemicin

-
-
34346-90-2
3-(3',4',5'-trimethoxyphenyl)-prop-2-en-1-al

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water Ambient temperature; | 80% |
-
-
487-11-6
elemicin

-
-
1373139-34-4
C12H16O6

| Conditions | Yield |
|---|---|
| With ozone | 80% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; tetramethyldisiloxane In ethanol at 20℃; for 24h; | 78% |
| Multi-step reaction with 2 steps 1: tetrabutylammomium bromide; potassium hydroxide / 0.67 h / 100 °C / Neat (no solvent) 2: pyridine; ozone / methanol; chloroform / -15 °C View Scheme | |
| Stage #1: elemicin With tetrabutylammomium bromide; potassium hydroxide at 100℃; for 0.666667h; Stage #2: With pyridine; ozone In methanol; chloroform at -15℃; | |
| Stage #1: elemicin With potassium hydroxide at 100℃; for 0.666667h; Stage #2: With ozone In pyridine; methanol; chloroform at 15℃; |

| Conditions | Yield |
|---|---|
| With potassium phosphate; cobalt(II) diacetate tetrahydrate In water; acetonitrile at 75℃; Schlenk technique; Inert atmosphere; | 78% |

-
-
487-11-6
elemicin

-
A
-
71277-13-9, 34346-90-2, 71277-12-8
3,4,5-trimethoxycinnamaldehyde

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In dichloromethane; water Ambient temperature; | A 71% B n/a C n/a |

-
-
487-11-6
elemicin

-
-
121637-68-1
1-(3,4,5-trimethoxyphenyl)-2-bromopropane

| Conditions | Yield |
|---|---|
| With hydrogen bromide In acetic acid at 0℃; for 3h; | 70% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate In water; acetonitrile at 80℃; for 24h; Sealed tube; | 70% |
| With dipotassium peroxodisulfate In water; acetonitrile at 80℃; for 24h; | 70% |


| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; trimethylsilylazide; manganese(III) triacetate dihydrate In water; acetonitrile at 45℃; Schlenk technique; Inert atmosphere; | 64% |
| With tert.-butylhydroperoxide; trimethylsilylazide; manganese(III) triacetate dihydrate In water; acetonitrile at 45℃; for 12h; Inert atmosphere; | 64% |

| Conditions | Yield |
|---|---|
| Stage #1: elemicin; triethylaluminum With zirconocene dichloride In toluene at 22℃; Inert atmosphere; Stage #2: With oxygen at 0℃; for 2h; | 60% |
-
-
487-11-6
elemicin

-
-
1278455-80-3
α-allyl,α-methyl nitroacetone

| Conditions | Yield |
|---|---|
| With water; palladium diacetate; caesium carbonate; triphenylphosphine; tert-butyl alcohol In dichloromethane; 1,2-dichloro-ethane at 80℃; for 22h; Schlenk technique; Inert atmosphere; | 57% |
-
-
487-11-6
elemicin

-
-
93-61-8
N-methyl-N-phenylformamide

-
-
71687-96-2
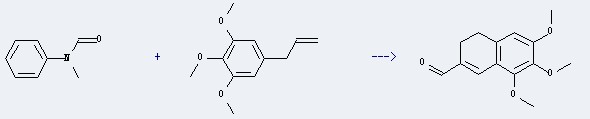
3,4-dihydro-6,7,8-trimethoxy-2-naphthalenecarboxaldehyde

| Conditions | Yield |
|---|---|
| With trichlorophosphate other methoxyallylbenzenes; | 56% |
| With trichlorophosphate 1) 10 deg C, 45 min 2) r.t., 24 h; Yield given. Multistep reaction; | |
| With trichlorophosphate 1) chlorobenzene, 2 h, 0 deg C, 2) chlorobenzene, 80 h, r.t.; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With oxygen; caesium carbonate In dimethyl sulfoxide at 120℃; under 760.051 Torr; for 24h; | 56% |

| Conditions | Yield |
|---|---|
| With water; oxygen; palladium diacetate; toluene-4-sulfonic acid In dimethyl sulfoxide at 110℃; under 760.051 Torr; for 24h; | 55% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; triphenylphosphine; silver carbonate In tetrahydrofuran Heck Reaction; | 50% |
-
-
487-11-6
elemicin

-
-
54306-10-4, 140385-37-1
3-(3',4',5'-trimethoxyphenyl)-1,2-propanediol

| Conditions | Yield |
|---|---|
| With potassium permanganate In ethanol; water for 0.05h; | 20% |
| With potassium permanganate |
-
-
487-11-6
elemicin

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
A
-
71687-96-2
3,4-dihydro-6,7,8-trimethoxy-2-naphthalenecarboxaldehyde

-
B
-
83923-94-8
6-allyl-2,3,4-trimethoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With trichlorophosphate In dichloromethane for 120h; Ambient temperature; | A 17% B 16.7% |

-
-
487-11-6
elemicin

-
-
38451-63-7
4,4a-dihydro-1,5,5,6,9,9-hexamethoxy-3,8a-bis(2-propenyl)-1,4-ethanonaphthalene-6,10(4H)-dione

-
A
-
6627-88-9
4-allyl-2,6-dimethoxyphenol

-
-
78660-19-2, 78660-20-5
demethylisoheterotropanone

-
-
70280-34-1, 70287-69-3
isoheterotropanone

-
-
70280-34-1, 70287-69-3
heterotropanone

| Conditions | Yield |
|---|---|
| at 175 - 180℃; for 1.5h; Further byproducts given; | A 3% B 2% C 13% D 14% |

-
-
487-11-6
elemicin

-
-
38451-63-7
4,4a-dihydro-1,5,5,6,9,9-hexamethoxy-3,8a-bis(2-propenyl)-1,4-ethanonaphthalene-6,10(4H)-dione

-
A
-
6627-88-9
4-allyl-2,6-dimethoxyphenol

-
-
78660-19-2, 78660-20-5
demethylheterotropanone

-
-
70280-34-1, 70287-69-3
isoheterotropanone

-
-
70280-34-1, 70287-69-3
heterotropanone

| Conditions | Yield |
|---|---|
| at 175 - 180℃; for 1.5h; Further byproducts given; | A 3% B 2% C 13% D 14% |

-
-
487-11-6
elemicin

-
-
41395-10-2
1,3-dimethoxy-5-propylbenzene

| Conditions | Yield |
|---|---|
| With ethanol; sodium |

| Conditions | Yield |
|---|---|
| With oxygen; ozone; ethyl acetate at -15℃; und anschliessende Hydrierung an Palladium/Calciumcarbonat unter Kuehlung; Isolierung als Natriumhydrogensulfit-Addukt; | |
| With water; ozone; benzene | |
| Multi-step reaction with 2 steps 1: KMnO4 2: HIO4; H2O View Scheme |
Elemicin Specification
The Elemicin, with the CAS registry number 487-11-6, is also known as 5-Allyl-1,2,3-trimethoxybenzene. Its molecular formula is C12H16O3 and its molecular weight is 208.2536. Additionally, its IUPAC is 1,2,3-trimethoxy-5-prop-2-enylbenzene. The classification code of this chemical is Mutation data.
Other characteristics of the Elemicin can be summarised as followings: (1)ACD/LogP: 2.68; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.68; (4)ACD/LogD (pH 7.4): 2.68; (5)ACD/BCF (pH 5.5): 63.54; (6)ACD/BCF (pH 7.4): 63.54; (7)ACD/KOC (pH 5.5): 679.55; (8)ACD/KOC (pH 7.4): 679.55; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 5; (12)Polar Surface Area: 27.69 Å2; (13)Index of Refraction: 1.496; (14)Molar Refractivity: 60.19 cm3; (15)Molar Volume: 205.8 cm3; (16)Polarizability: 23.86×10-24cm3; (17)Surface Tension: 30.2 dyne/cm; (18)Density: 1.011 g/cm3; (19)Flash Point: 92.6 °C; (20)Enthalpy of Vaporization: 49.77 kJ/mol; (21)Boiling Point: 279.8 °C at 760 mmHg; (22)Vapour Pressure: 0.00666 mmHg at 25°C.
Uses of the Elemicin: It could react with N-methyl-N-phenyl-formamide to obtain the 6,7,8-Trimethoxy-3,4-dihydro-2-naphthalincarbaldehyd. This reaction needs the reagent of POCl3. The yield is 56 %. In addition, this reaction needs the other methoxyallylbenzenes.
You can still convert the following datas into molecular structure:
(1)SMILES: O(c1cc(cc(OC)c1OC)C\C=C)C
(2)InChI: InChI=1/C12H16O3/c1-5-6-9-7-10(13-2)12(15-4)11(8-9)14-3/h5,7-8H,1,6H2,2-4H3
(3)InChIKey: BPLQKQKXWHCZSS-UHFFFAOYAD
(4)Std. InChI: InChI=1S/C12H16O3/c1-5-6-9-7-10(13-2)12(15-4)11(8-9)14-3/h5,7-8H,1,6H2,2-4H3
(5)Std. InChIKey: BPLQKQKXWHCZSS-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View