-
Name
Ethanesulfonic acid
- EINECS 209-843-0
- CAS No. 594-45-6
- Article Data79
- CAS DataBase
- Density 1.375 g/cm3
- Solubility soluble
- Melting Point -17 °C(lit.)
- Formula C2H6O3S
- Boiling Point 394.9 °C at 760 mmHg
- Molecular Weight 110.134
- Flash Point >230 °F
- Transport Information UN 2586 8/PG 3
- Appearance Yellow To Brown Liquid
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 1-Ethanesulfonicacid;Ethanesulphonic acid;
- PSA 62.75000
- LogP 0.97490
Synthetic route

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methyltrioxorhenium(VII) In acetonitrile at 20℃; for 0.0166667h; | 80% |
| With sodium hypochlorite bei der Oxydation; | |
| With bromine | |
| With air; nitric acid; Nitrogen dioxide | |
| Anodische Oxydation; |
-
-
10467-10-4
ethylmagnesium iodide

-
-
594-45-6
ethanesulfonic acid

| Conditions | Yield |
|---|---|
| With diethyl ether; sulfur dioxide Zersetzen des Reaktionsproduktes mit Wasser und Oxydieren der waessr.Loesung mit Brom; |

| Conditions | Yield |
|---|---|
| With ammonium sulfite |

| Conditions | Yield |
|---|---|
| With nitric acid erst in der Kaelte dann in der Waerme; |
-
-
26185-93-3
S-nitrosoethanethiol

-
-
594-45-6
ethanesulfonic acid

| Conditions | Yield |
|---|---|
| With oxygen |

| Conditions | Yield |
|---|---|
| With alkali halide | |
| With alkali thiocyanate | |
| With potassium hydroxide | |
| With tributyl-amine at 150 - 160℃; unter Abdestillieren niedrigsiedender reaktionsprodukte; |

| Conditions | Yield |
|---|---|
| With ammonium hydrogen sulfite; water | |
| With ammonium disulfite | |
| With sulfur dioxide In water in presence of HNO3 or nitrates, nitrites, perchloric acid or perchlorates or K2Cr2O7, below 200°C, alkali hydrogen sulphites also used istead of SO2; | |
| With SO2 In water in presence of HNO3 or nitrates, nitrites, perchloric acid or perchlorates or K2Cr2O7, below 200°C, alkali hydrogen sulphites also used istead of SO2; |

| Conditions | Yield |
|---|---|
| With nitric acid | |
| With sodium hypochlorite | |
| With hydrogenchloride; acetic acid at 15 - 20℃; elektrolytische Oxydation; |

| Conditions | Yield |
|---|---|
| With phosphorus; hydrogen iodide at 170℃; |

| Conditions | Yield |
|---|---|
| With alkali halide | |
| With alkali thiocyanate |

| Conditions | Yield |
|---|---|
| With water; sodium sulfite at 110 - 120℃; | |
| With water; sodium sulfite at 110 - 120℃; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With ammonium sulfite; water | |
| With potassium sulfite at 130 - 150℃; | |
| With sodium sulfite at 130 - 150℃; |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite |

| Conditions | Yield |
|---|---|
| With potassium permanganate | |
| With bromine | |
| With nitric acid |

-
-
594-45-6
ethanesulfonic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
19157-19-8
1,1-bis-ethanesulfonyl-but-1-ene

-
-
594-45-6
ethanesulfonic acid

| Conditions | Yield |
|---|---|
| With ozone In chloroform |
-
-
67-56-1
methanol

-
-
32317-03-6
Methylthiophosphonsaeure-O-isopropylester-S-ethylester

-
A
-
690-64-2
isopropyl methyl methylphosphonate

-
B
-
594-45-6
ethanesulfonic acid

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid |


| Conditions | Yield |
|---|---|
| With water In dimethyl sulfoxide at 37℃; Rate constant; phosphate buffer, pH = 7.4; |

| Conditions | Yield |
|---|---|
| With water at 25℃; Rate constant; 0.1 M KCl; | |
| With potassium chloride at 25℃; Rate constant; in the presence of triethylamine in water or triethylamine, 2-propanol in methylene chloride; |
-
-
57557-80-9
O,S-diethyl phenylphosphonothionate

-
A
-
594-45-6
ethanesulfonic acid

-
B
-
4546-19-4
ethyl hydrogen phenylphosphonate

| Conditions | Yield |
|---|---|
| With Oxone In water; acetonitrile at 25 - 50℃; Kinetics; Mechanism; Thermodynamic data; ΔH excit., ΔS excit., solvent deuterium isotope effect; |
-
-
3096-04-6
S-ethyl diphenylphosphorothiolate

-
A
-
598-02-7
Diethyl phosphate

-
B
-
594-45-6
ethanesulfonic acid

| Conditions | Yield |
|---|---|
| With Oxone In water; acetonitrile at 25℃; Rate constant; Mechanism; solvent deuterium isotope effect; |


| Conditions | Yield |
|---|---|
| In acetonitrile for 1h; Ambient temperature; | 100% |
-
-
656247-17-5
nintedanib

-
-
594-45-6
ethanesulfonic acid

-
-
656247-18-6
methyl (3Z)-3-[({4-[N-methyl-2-(4-methylpiperazin-1-yl)acetamido]phenyl}amino)(phenyl)methylidene]-2-oxo-2,3-dihydro-1H-indole-6-carboxylate ethanesulfonate salt

| Conditions | Yield |
|---|---|
| In methanol for 4h; Reflux; | 100% |
| In methanol; water at 60℃; | 97.3% |
| In methanol; water; isopropyl alcohol at 0 - 65℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| Inert atmosphere; | 100% |
-
-
594-45-6
ethanesulfonic acid

-
-
10424-65-4
tetramethylammonium hydroxide pentahydrate

| Conditions | Yield |
|---|---|
| In methanol Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In methanol Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In methanol Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In methanol Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In methanol Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In methanol Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol Inert atmosphere; | 100% |
-
-
594-45-6
ethanesulfonic acid

-
-
1187594-09-7
Baricitinib

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20 - 22℃; for 2h; Solvent; Temperature; | 98.8% |

-
-
594-45-6
ethanesulfonic acid

-
-
1614220-86-8
dodecyl 2-(dimethylamino)propanoate ethanesulfonate

| Conditions | Yield |
|---|---|
| In ethyl acetate at 0 - 20℃; for 12h; | 98.4% |
-
-
594-45-6
ethanesulfonic acid


| Conditions | Yield |
|---|---|
| In acetonitrile at 5 - 55℃; for 24h; Inert atmosphere; | 98% |
-
-
1004-36-0
2,6-dimethylpyrone

-
-
594-45-6
ethanesulfonic acid

-
-
1325228-59-8
4-hydroxy-2,6-dimethylpyrylium ethanesulfonate

| Conditions | Yield |
|---|---|
| In methanol for 1h; Reflux; | 97% |
-
-
594-45-6
ethanesulfonic acid

-
-
714971-09-2
[4-[[1-(3-fluorophenyl)methyl]-1H-indazol-5-ylamino]-5-methyl-pyrrolo[2,1-f][1,2,4]triazin-6-yl]carbamic acid (3S)-3-morpholinylmethyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol; tert-butyl methyl ether at 30 - 40℃; Product distribution / selectivity; | 97% |
-
-
594-45-6
ethanesulfonic acid

-
-
70284-09-2
1-ethanesulfonyl azide

| Conditions | Yield |
|---|---|
| Stage #1: ethanesulfonic acid With trichloroisocyanuric acid; triphenylphosphine In tetrahydrofuran at 0 - 5℃; Stage #2: With sodium azide In tetrahydrofuran at 5 - 20℃; | 96% |
| With sodium azide; trichloroacetonitrile; triphenylphosphine In acetonitrile at 20℃; for 2h; | 83% |
| With sodium azide; trichloroisocyanuric acid; triphenylphosphine In tetrahydrofuran at 20℃; |
-
-
594-45-6
ethanesulfonic acid

-
-
1044642-88-7
tegaserod

| Conditions | Yield |
|---|---|
| In acetone at 25 - 50℃; for 20h; | 95.6% |

| Conditions | Yield |
|---|---|
| In water | 95% |


| Conditions | Yield |
|---|---|
| In water | 95% |


| Conditions | Yield |
|---|---|
| In water | 95% |


| Conditions | Yield |
|---|---|
| In water | 95% |


| Conditions | Yield |
|---|---|
| In water | 95% |

-
-
594-45-6
ethanesulfonic acid

-
-
1206161-97-8
N-(5-{4-[(1,1-dioxo-1λ6-thiomorpholin-4-yl)methyl]phenyl}-[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanecarboxamide

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 80℃; for 1.66667h; Time; | 95% |

| Conditions | Yield |
|---|---|
| at 110℃; for 12h; Sealed tube; | 95% |

-
-
864740-19-2
(2R,4aR,10bR)-6-(2,6-dimethoxy-pyridin-3-yl)-9-ethoxy-8-methoxy-1,2,3,4,4a,10b-hexahydro-phenanthridin-2-ol

-
-
594-45-6
ethanesulfonic acid

-
-
909115-55-5
(2R,4aR,10bR)-6-(2,6-dimethoxy-pyridin-3-yl)-9-ethoxy-8-methoxy-1,2,3,4,4a,10b-hexahydro-phenanthridin-2-ol ethansulfonate

| Conditions | Yield |
|---|---|
| In 4-methyl-2-pentanone at 50℃; | 94% |
-
-
847453-47-8
(2R,3R,4R,5R)-2-(hydroxymethyl)-5-(5-amino-2-oxothiazolo[4,5-d]pyrimidin-3(2H)-yl)tetrahydrofuran-3,4-diyl diacetate

-
-
594-45-6
ethanesulfonic acid

-
-
1106672-27-8
5-amino-3-(2',3'-di-O-acetyl-beta-D-ribofuranosyl)-3H-thiazolo[4,5-d]pyrimidin-2-one esylate

| Conditions | Yield |
|---|---|
| In butanone at 0 - 50℃; | 93.27% |
-
-
51608-61-8, 13707-44-3
N-(2,2,2-trichloroethyliden)-p-toluenesulfonamide

-
-
594-45-6
ethanesulfonic acid

| Conditions | Yield |
|---|---|
| With (methoxymethylidene)dimethylammonium methyl sulfate; triethylamine In tetrahydrofuran at 20℃; for 4h; diastereoselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| at 110℃; for 12h; | 93% |

-
-
594-45-6
ethanesulfonic acid

-
-
105889-80-3
((6R,7R)-3-((aminocarbonyl)oxy)methyl-7-((Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoyl)amino)-8-oxo-5-thio-1-azabicyclo[4.2.0]-oct-2-ene-2-carboxylic acid(2,2-dimethyloxypropoxymethyl)ester

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 16h; | 92.3% |
-
-
861151-12-4
5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-N-piperidin-1-yl-4,5-dihydro-1H-pyrazole-3-carboxamide

-
-
594-45-6
ethanesulfonic acid

| Conditions | Yield |
|---|---|
| In ethyl acetate at 3 - 70℃; for 24h; Product distribution / selectivity; | 92% |
| In pentanone, 3- at 3 - 95℃; for 24h; Product distribution / selectivity; | 92% |
Ethanesulfonic acid Specification
The IUPAC name of this chemical is Ethanesulfonic acid. With the CAS registry number 594-45-6 and EINECS registry number 209-843-0, it is also named as Ethanesulphonic acid. In addition, the molecular formula is C2H6O3S and the molecular weight is 110.13. It is a kind of clear yellow to brown liquid and belongs to the classes of Pharmaceutical Intermediates; Organic Building Blocks; Sulfonic/Sulfinic Acids; Sulfur Compounds; Others; Supported Reagents; Supported Synthesis.
Physical properties about this chemical are: (1)ACD/LogP: -1.36; (2)ACD/LogD (pH 5.5): -4.64; (3)ACD/LogD (pH 7.4): -4.86; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 3; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Polar Surface Area: 51.75 Å2; (12)Index of Refraction: 1.453; (13)Molar Refractivity: 21.65 cm3; (14)Molar Volume: 80 cm3; (15)Polarizability: 8.58 ×10-24cm3; (16)Surface Tension: 48.7 dyne/cm; (17)Density: 1.375 g/cm3; (18)Boiling Point: 394.9 °C at 760 mmHg; (19)Vapour Pressure: 2.45E-07 mmHg at 25°.
Uses of Ethanesulfonic acid: it can be used as catalysts for alkylation, polymerization and other reaction. And it can react with triethoxymethane to get ethanesulfonic acid ethyl ester. The reaction time is 14 hours with ambient temperature. The yield is about 73%.
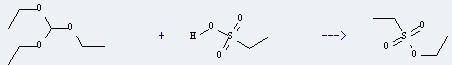
When you are using this chemical, please be cautious about it as the following:
This chemical can cause burns. During using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.).
You can still convert the following datas into molecular structure:
(1)SMILES: O=S(=O)(O)CC
(2)InChI: InChI=1/C2H6O3S/c1-2-6(3,4)5/h2H2,1H3,(H,3,4,5)
(3)InChIKey: CCIVGXIOQKPBKL-UHFFFAOYAI
Related Products
- Ethanesulfonic acid
- Ethanesulfonic acid, 2-[(2-aminoethyl)amino]-, sodium salt (1:1)
- Ethanesulfonic acid, 2-hydroxy-, compd. with 9-((2-methoxy-4-((methylsulfonyl)amino)phenyl)amino)-N,5-dimethyl-4-
- Ethanesulfonic acid, methyl ester
- Ethanesulfonic acid,2,2'-dithiobis-
- Ethanesulfonic acid,2-hydroxy-, potassium salt (1:1)
- 5944-61-6
- 59447-06-2
- 59447-12-0
- 59447-55-1
- 59447-77-7
- 59452-49-2
- 5945-33-5
- 59454-35-2
- 5945-50-6
- 59455-91-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View