-
Name
Flufenamic acid
- EINECS 208-494-1
- CAS No. 530-78-9
- Article Data29
- CAS DataBase
- Density 1.396 g/cm3
- Solubility 0.0265 g/L (37 ºC)
- Melting Point 132-135 °C(lit.)
- Formula C14H10F3NO2
- Boiling Point 373.903 °C at 760 mmHg
- Molecular Weight 281.234
- Flash Point 179.93 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance pale-yellow solid
- Safety 26
- Risk Codes 22-36/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Anthranilicacid, N-(a,a,a-trifluoro-m-tolyl)- (6CI,8CI);2-[[3-(Trifluoromethyl)phenyl]amino]benzoic acid;3'-Trifluoromethyldiphenylamine-2-carboxylic acid;Achless;Arlef;Fullsafe;Meralen;Movilizin;N-(a,a,a-Trifluoro-m-tolyl)anthranilic acid;N-[3-(Trifluoromethyl)phenyl]anthranilic acid;Pinox;
- PSA 49.33000
- LogP 4.22020
Synthetic route

| Conditions | Yield |
|---|---|
| With human plasma In phosphate buffer at 37℃; pH=7.4; | A n/a B 100% |

-
A
-
34105-57-2
2-amino-N,N-diethylacetamide

-
B
-
530-78-9
flufenamic acid

| Conditions | Yield |
|---|---|
| With human plasma In phosphate buffer at 37℃; pH=7.4; | A n/a B 100% |

| Conditions | Yield |
|---|---|
| With human plasma In phosphate buffer at 37℃; pH=7.4; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| With human plasma In phosphate buffer at 37℃; pH=7.4; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| With human plasma In phosphate buffer at 37℃; pH=7.4; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| With human plasma In phosphate buffer at 37℃; pH=7.4; | A 100% B n/a |
-
-
13481-62-4
2-((3-(trifluoromethyl)phenyl)amino)benzonitrile

-
-
530-78-9
flufenamic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water at 95℃; for 20h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; copper In N,N-dimethyl-formamide for 2h; Heating; | 60% |
| With copper(l) iodide; copper; potassium carbonate In N,N-dimethyl-formamide for 0.333333h; Heating; ultrasonic irradiation; | 24% |
| (i) aq. KOH, (ii) /BRN= 387672/, Cu; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With water; potassium hydroxide In ethanol at 100℃; Inert atmosphere; | 55% |
| Stage #1: 2-[[3-(trifluoromethyl)phenyl]amino]benzoic acid,methyl ester With potassium hydroxide In ethanol; water at 20 - 100℃; Inert atmosphere; Stage #2: With hydrogenchloride In water at 0℃; pH=2; Inert atmosphere; | 55% |
| With water; potassium hydroxide In ethanol at 100℃; | 55% |
| With hydrazine hydrate In methanol |

| Conditions | Yield |
|---|---|
| With water; copper; potassium carbonate | |
| With 4-methyl-morpholine; copper(I) oxide In 1,4-dioxane at 100℃; for 3h; Ullmann Condensation; Inert atmosphere; |
-
-
32509-03-8
1-(3'-trifluoromethyl-phenyl)-1,2-dihydro-3,1-benzoxazin-4-one

-
-
530-78-9
flufenamic acid

| Conditions | Yield |
|---|---|
| With water In methanol at 20℃; Rate constant; pH from 2.0 to 8.0, half-live-time; |

| Conditions | Yield |
|---|---|
| With N-ethylmorpholine;; copper diacetate In N,N-dimethyl-formamide at 145℃; for 3h; |
-
-
137488-39-2
1-(3'-trifluoromethyl-phenyl)-2-chloromethyl-1,2-dihydro-3,1-benzoxazin-4-one

-
-
530-78-9
flufenamic acid

| Conditions | Yield |
|---|---|
| With water In methanol at 20℃; Rate constant; pH from 2.0 to 9.0, half-live-time; |
-
-
137488-40-5
1-(3'-trifluoromethyl-phenyl)-2-methoxymethyl-1,2-dihydro-3,1-benzoxazin-4-one

-
-
530-78-9
flufenamic acid

| Conditions | Yield |
|---|---|
| With water In methanol at 20℃; Rate constant; pH from 2.0 to 9.0, half-live-time; |
-
-
137488-41-6
1-(3'-trifluoromethyl-phenyl)-2-ethoxycarbonyl-1,2-dihydro-3,1-benzoxazin-4-one

-
-
530-78-9
flufenamic acid

| Conditions | Yield |
|---|---|
| With water In methanol at 37℃; Rate constant; pH from 2.0 to 9.0, half-live-time; |
-
-
137138-25-1
2-<<3-(Trifluormethyl)phenyl>amino>benzoeaeure-2-oxopropylester

-
-
530-78-9
flufenamic acid

| Conditions | Yield |
|---|---|
| With buffer pH 8.0 In 1,4-dioxane; acetonitrile at 57℃; half-life time of hydrolysis at different pH; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 39 percent / tetrabutylammonium bromide; Et3N / tetrahydrofuran / 24 h / 20 °C 2: 100 percent / human plasma / aq. phosphate buffer / 37 °C / pH 7.4 View Scheme |
-
-
17763-70-1
methyl 2-(trifluoromethylsulfonyloxy)benzoate

-
-
98-16-8
3-trifluoromethylaniline

-
-
530-78-9
flufenamic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl / toluene / 120 °C / Inert atmosphere 2: water; potassium hydroxide / ethanol / 100 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1.1: palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl / toluene / 20 - 120 °C / Inert atmosphere 2.1: potassium hydroxide / ethanol; water / 20 - 100 °C / Inert atmosphere 2.2: 0 °C / pH 2 / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl / toluene / 120 °C 2: water; potassium hydroxide / ethanol / 100 °C View Scheme |

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; copper(II) oxide In 1,4-dioxane Inert atmosphere; Reflux; |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With triethylsilane; palladium diacetate; 2,2-dimethylpropanoic anhydride; 1,4-di(diphenylphosphino)-butane In toluene at 160℃; for 15h; chemoselective reaction; | 97% |
-
-
530-78-9
flufenamic acid

-
-
39492-79-0
2-(3-(trifluoromethyl)phenylamino)benzohydrazide

| Conditions | Yield |
|---|---|
| With hydrazine hydrate at 250℃; under 12929 Torr; for 0.0666667h; Microwave irradiation; Neat (no solvent); | 96% |
| Multi-step reaction with 2 steps 1: sulfuric acid / Reflux 2: hydrazine hydrate / 15 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With palladium diacetate; 2,2-dimethylpropanoic anhydride; triethylamine; 1,4-di(diphenylphosphino)-butane In 1,4-dioxane at 160℃; for 15h; Inert atmosphere; Schlenk technique; | 96% |
-
-
50-00-0
formaldehyd

-
-
530-78-9
flufenamic acid

-
-
32509-03-8
1-(3'-trifluoromethyl-phenyl)-1,2-dihydro-3,1-benzoxazin-4-one

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In dichloromethane for 2h; Ambient temperature; | 93% |
| In ethanol; water for 1h; Heating; |
-
-
530-78-9
flufenamic acid

-
-
95-53-4
o-toluidine

-
-
21240-02-8
3-Trifluormethyl-2'-(2-methyl-phenylcarbamoyl)-diphenylamin

| Conditions | Yield |
|---|---|
| With N,N-bis[2-oxo-3-oxazolidinyl]phosphorodiamidic chloride; triethylamine In dichloromethane at 20 - 25℃; for 1h; | 92% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 4h; | 92% |
-
-
530-78-9
flufenamic acid

-
-
13481-61-3
2-<<3-(trifluoromethyl)phenyl>amino>benzamide

| Conditions | Yield |
|---|---|
| Stage #1: flufenamic acid With thionyl chloride In toluene for 1h; Reflux; Stage #2: With ammonium hydroxide | 92% |
| Stage #1: flufenamic acid With 1,1'-carbonyldiimidazole In tetrahydrofuran at 50℃; for 0.5h; Stage #2: With ammonium hydroxide; 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 50℃; for 5h; | 70.3% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 1h; | 91% |
| With dmap In dichloromethane at 25 - 30℃; | 91% |
-
-
530-78-9
flufenamic acid

-
-
122-51-0
orthoformic acid triethyl ester

-
-
137488-38-1
1-(3'-trifluoromethyl-phenyl)-2-ethoxy-1,2-dihydro-3,1-benzoxazin-4-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 4h; Heating; | 89% |
-
-
530-78-9
flufenamic acid

-
-
149-73-5
trimethyl orthoformate

-
-
137488-37-0
1-(3'-trifluoromethyl-phenyl)-2-methoxy-1,2-dihydro-3,1-benzoxazin-4-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 4h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: captamine; flufenamic acid With dicyclohexyl-carbodiimide In chloroform at 20℃; for 3h; Stage #2: acetic acid In chloroform | 88.5% |

| Conditions | Yield |
|---|---|
| Stage #1: flufenamic acid With oxalyl dichloride In dichloromethane Stage #2: thiophenol With triethylamine In dichloromethane | 88% |
-
-
530-78-9
flufenamic acid

-
-
134674-19-4
R,S-5-Brom-2,2-dimethyl-1,3-dioxolan-4-on

-
-
134674-24-1
2-<3-(Trifluormethyl)phenylamino>-benzoesaeure-(2,2-dimethyl-1,3-dioxolan-4-on-5-yl)ester

| Conditions | Yield |
|---|---|
| With triethylamine In acetone Ambient temperature; | 87% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide at 20℃; for 4h; | 87% |
-
-
67-56-1
methanol

-
-
530-78-9
flufenamic acid

-
-
2765-91-5
2-[[3-(trifluoromethyl)phenyl]amino]benzoic acid,methyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid for 12h; Heating; | 86% |
| With sulfuric acid for 100h; Heating; | |
| With sulfuric acid for 16h; Heating; | |
| With sulfuric acid Reflux; |
-
-
97-97-2
chloroacetaldehyde dimethyl acetal

-
-
530-78-9
flufenamic acid

-
-
137488-39-2
1-(3'-trifluoromethyl-phenyl)-2-chloromethyl-1,2-dihydro-3,1-benzoxazin-4-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 4h; Heating; | 86% |
-
-
530-78-9
flufenamic acid

-
-
6065-82-3
Ethyl diethoxyacetate

-
-
137488-41-6
1-(3'-trifluoromethyl-phenyl)-2-ethoxycarbonyl-1,2-dihydro-3,1-benzoxazin-4-one

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 5h; Heating; | 86% |
Flufenamic acid Chemical Properties
Chemical Name: Flufenamic acid
IUPAC NAME: 2-[3-(Trifluoromethyl)anilino]benzoic acid
CAS No.: 530-78-9
EINECS: 208-494-1
RTECS: CB4375000
RTECS Class: Drug ; Mutagen ; Reproductive Effector ; Human Data
Molecular Formula: C14H10F3NO2
Molecular Weight: 281.23 g/mol
Melting Point: 132-135 °C(lit.)
Density: 1.395 g/cm3
Flash Point: 179.9 °C
Boiling Point: 373.9 °C at 760 mmHg
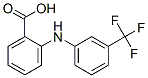
Following is the structure of N-(α,α,α-TRIFLUORO-m-TOLYL)ANTHRANILIC ACID (530-78-9):
Product Categories about N-(α,α,α-TRIFLUORO-m-TOLYL)ANTHRANILIC ACID (530-78-9) are Active Pharmaceutical Ingredients ; Aromatics ; Intermediates & Fine Chemicals ; Pharmaceuticals ; API's
The chemical synonymous of N-(α,α,α-TRIFLUORO-m-TOLYL)ANTHRANILIC ACID (530-78-9) are Inf1837 ; Lanceat ; Meralen ; N-((M-Trifluoromethyl)phenyl)-2-Aminobenzoic acid ; N-(α,α,α-trifluoro-m-tolyl)anthranilate ; N-(α,α,α-trifluoro-m-tolyl)-anthranilicaci ; N-(M-Trifluoromethylphenyl)-2-aminobenzoicacid ; Nichisedan
Flufenamic acid Uses
N-(α,α,α-TRIFLUORO-m-TOLYL)ANTHRANILIC ACID (530-78-9) is used as an anthranilic acid derivative with analgesic, anti-inflammatory, and antipyretic properties.
Flufenamic acid Toxicity Data With Reference
| 1. | dni-hmn-unr 504 mg/kg/8W | STBIBN Studia Biophysica. 50 (1975),172. | ||
| 2. | orl-wmn TDLo:2160 mg/kg/26W-I:GIT | PGMJAO Postgraduate Medical Journal. 62 (1986),773. | ||
| 3. | orl-rat LD50:249 mg/kg | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 221 (1976),132. | ||
| 4. | ipr-rat LD50:185 mg/kg | OYYAA2 Oyo Yakuri. Pharmacometrics. 16 (1978),1011. | ||
| 5. | ivn-rat LD50:98 mg/kg | CMROCX Current Medical Research and Opinion. 4 (1976),17. | ||
| 6. | orl-mus LD50:490 mg/kg | OYYAA2 Oyo Yakuri. Pharmacometrics. 16 (1978),1011. | ||
| 7. | ipr-mus LD50:150 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD691-490 . | ||
| 8. | ivn-mus LD50:158 mg/kg | YKKZAJ Yakugaku Zasshi. Journal of Pharmacy. 89 (1969),1392. |
Flufenamic acid Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Experimental reproductive effects. Human systemic effects: hypermotility, diarrhea. Human mutation data reported. When heated to decomposition it emits very toxic fumes of F− and NOx. Used as an anti-inflammatory agent. See also FLUORIDES.
Hazard Codes:
Xi: Irritant ![]()
Risk Statements about N-(α,α,α-TRIFLUORO-m-TOLYL)ANTHRANILIC ACID (530-78-9):
R21/22 Harmful in contact with skin and if swallowed.
R36/37/38 Irritating to eyes, respiratory system and skin.
Safety Statements about N-(α,α,α-TRIFLUORO-m-TOLYL)ANTHRANILIC ACID (530-78-9):
S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39 Wear suitable protective clothing, gloves and eye/face protection.
Attentions:
1. Storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
2. Handling: Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View